B9602
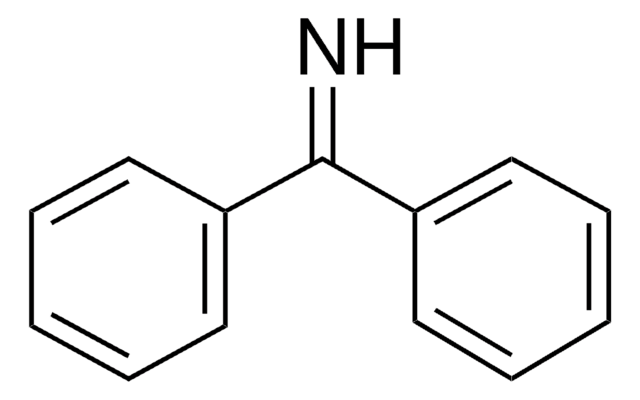
Benzophenone hydrazone
96%
Synonym(s):
Diphenyldiazomethane precursor
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C6H5)2C=NNH2
CAS Number:
Molecular Weight:
196.25
Beilstein/REAXYS Number:
1910177
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
crystals
bp
225-230 °C/55 mmHg (lit.)
mp
95-98 °C (lit.)
Storage temp.
2-8°C
SMILES string
N\N=C(/c1ccccc1)c2ccccc2
InChI
1S/C13H12N2/c14-15-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,14H2
Inchi Key
QYCSNMDOZNUZIT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
294.8 °F
flash_point_c
146 °C
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and urease inhibitory potential of benzophenone sulfonamide hybrid in vitro and in silico.
Arshia et al.
Bioorganic & medicinal chemistry, 27(6), 1009-1022 (2019-02-11)
This study deals with the synthesis of benzophenone sulfonamides hybrids (1-31) and screening against urease enzyme in vitro. Studies showed that several synthetic compounds were found to have good urease enzyme inhibitory activity. Compounds 1 (N'-((4'-hydroxyphenyl)(phenyl)methylene)-4''-nitrobenzenesulfonohydrazide), 2 (N'-((4'-hydroxyphenyl)(phenyl)methylene)-3''-nitrobenzenesulfonohydrazide), 3 (N'-((4'-hydroxyphenyl)(phenyl)methylene)-4''-methoxybenzenesulfonohydrazide)
Fatemeh M Mir et al.
Journal of medicinal chemistry, 62(9), 4500-4525 (2019-04-02)
Peptide mimicry employing a combination of aza-amino acyl proline and indolizidinone residues has been used to develop allosteric modulators of the prostaglandin F2α receptor. The systematic study of the N-terminal phenylacetyl moiety and the conformation and side chain functions of
Kelvine Chignen Possi et al.
Journal of medicinal chemistry, 60(22), 9263-9274 (2017-10-14)
Azapeptide analogues of growth hormone releasing peptide-6 (GHRP-6) exhibit promising affinity, selectivity, and modulator activity on the cluster of differentiation 36 receptor (CD36). For example, [A
Ngoc-Duc Doan et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(5), 387-391 (2014-11-18)
The solid-phase synthesis of azapeptides possessing a C-terminal aza-residue has been accomplished by a protocol featuring regioselective alkylation of benzhydrylidene-aza-glycinamide and illustrated by the syntheses of [aza-Lys(6)] growth-hormone-releasing peptide-6 analogs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service