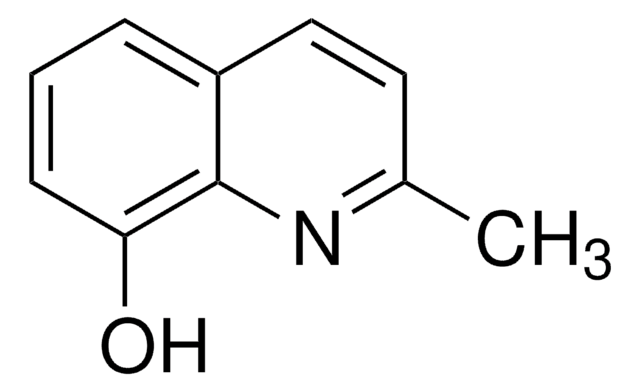
H43806
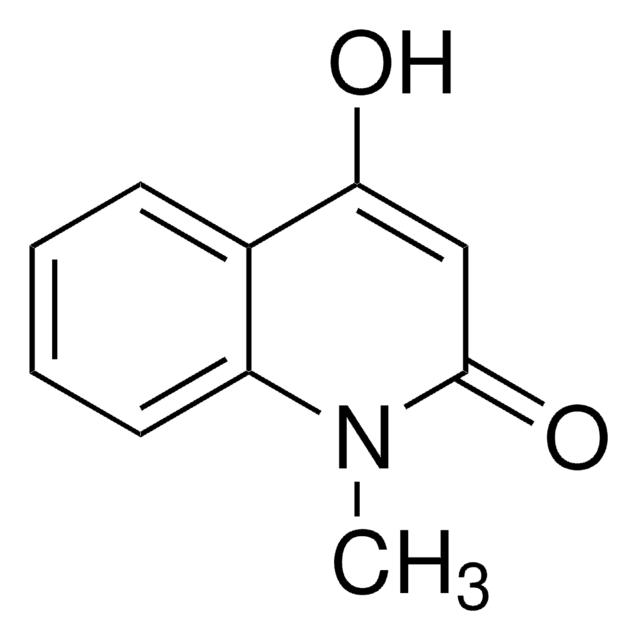
4-Hydroxy-2-methylquinoline
98.5%
Synonym(s):
2-Methyl-4-quinolinol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98.5%
SMILES string
Cc1cc(O)c2ccccc2n1
InChI
1S/C10H9NO/c1-7-6-10(12)8-4-2-3-5-9(8)11-7/h2-6H,1H3,(H,11,12)
InChI key
NWINIEGDLHHNLH-UHFFFAOYSA-N
Application
4-Hydroxy-2-methylquinoline can be used as an intermediate in the synthesis of a wide range of medicinally important compounds such as:
- Synthesis of 2-(quinolin-4-yloxy)acetamides as potent antitubercular agents.
- Synthesis of 2-arylethenylquinoline derivatives for the treatment of Alzheimer′s disease.
- Synthesis of 1,10-diethoxy-1H-pyrano[4,3-b]quinolones as antibacterial agents.
- Synthesis of phenylimidazole-pyrazolo[1,5-c]quinazolines as potent phophodiesterase 10A (PDE10A) inhibitors.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2-(Quinolin-4-yloxy) acetamides are active against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains.
Pissinate K, et al.
ACS Medicinal Chemistry Letters, 7(3), 235-239 (2016)
Synthesis of new 1, 10-diethoxy-1H-pyrano [4, 3-b] quinolines and their antibacterial studies.
Dhanabal T, et al.
Indian J. Chem. B, 45B(02) (2006)
Design, synthesis, and biological evaluation of 2-arylethenylquinoline derivatives as multifunctional agents for the treatment of Alzheimer's disease.
Wang X Q, et al.
European Journal of Medicinal Chemistry, 89, 349-361 (2015)
Quinaldine derivatives: Preparation and biological activity.
Jampilek J, et al.
Medicinal Chemistry, 1(6), 591-599 (2005)
Synthesis and SAR study of new phenylimidazole-pyrazolo [1, 5-c] quinazolines as potent phosphodiesterase 10A inhibitors.
Asproni B, et al.
Bioorganic & Medicinal Chemistry, 19(1), 642-649 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service