All Photos(1)
About This Item
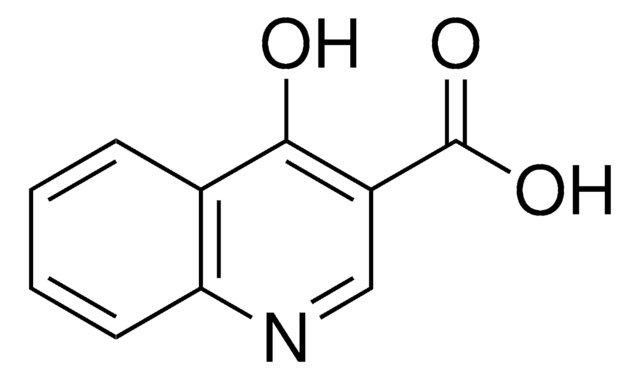
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein/REAXYS Number:
2900
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
200-202 °C (lit.)
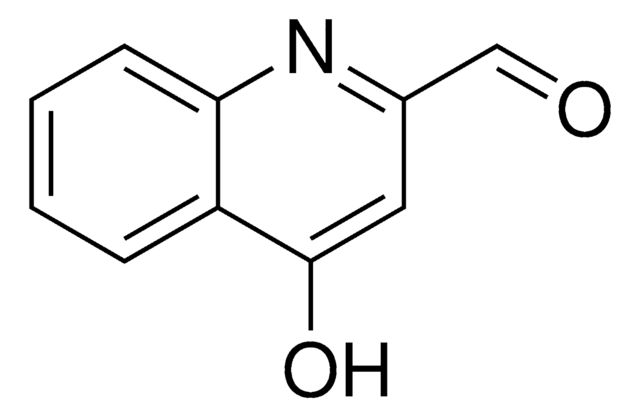
SMILES string
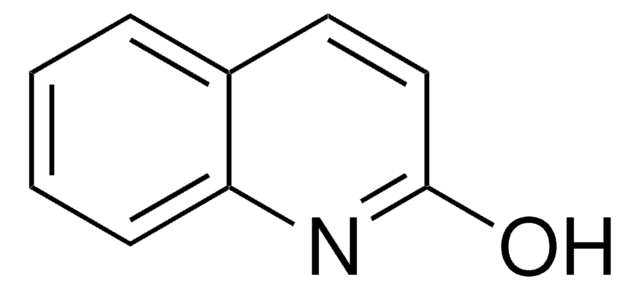
Oc1ccnc2ccccc12
InChI
1S/C9H7NO/c11-9-5-6-10-8-4-2-1-3-7(8)9/h1-6H,(H,10,11)
InChI key
PMZDQRJGMBOQBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Quinolinol (4-quinolone) is a quinolone compound which forms the core moiety of antibacterials such as norfloxacin, nalidixic acid, ciprofloxacin and cinoxacin.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Josip Podobnik et al.
Biomolecules, 10(11) (2020-11-19)
Juvenile delinquency is related to several biological factors, yet very few vulnerability biomarkers have been identified. Previous data suggest that the enzyme monoamine oxidase B (MAO-B) influences several personality traits linked to the propensity to engage in delinquent behavior. Building
Michail N Elinson et al.
Molecular diversity, 14(4), 833-839 (2009-11-19)
Electrochemically induced catalytic multicomponent transformation of isatins, 4-hydroxyquinolin-2(1H)-one and malononitrile in ethanol in an undivided cell in the presence of sodium bromide as an electrolyte results in the formation of spirooxindoles with fused functionalized indole-3,4'-pyrano[3,2-c]quinoline] scaffold in 75-91% substance yields
Zhengyin Yan et al.
Rapid communications in mass spectrometry : RCM, 18(8), 834-840 (2004-04-20)
A highly efficient method utilizing liquid chromatography with tandem mass spectrometry (LC/MS/MS) was developed and employed for high-throughput screening of compounds for monoamine oxidase (MAO) inhibition. The method used kynuramine as a common substrate for both MAO-A and MAO-B in
Takeshi Fuchigami et al.
Nuclear medicine and biology, 35(2), 203-212 (2008-03-04)
High-affinity iodine- and ethyl-C-5 substituted analogs of 4-hydroxy-3-(3-[11C]methoxyphenyl)-2(1H)-quinolone ([11C]4HQ) were synthesized as new positron emission tomography radioligands for the glycine-binding sites of the N-methyl-d-aspartate (NMDA) ion channel. Although both radioligands showed high in vitro specific binding to rat brain slices
G C Ragos et al.
Farmaco (Societa chimica italiana : 1989), 53(8-9), 611-616 (1999-03-19)
A new sensitive, rapid and accurate spectrofluorimetric method, suitable for the determination of micromolar concentrations of iron(III) in bovine liver, using 4-hydroxyquinoline (4-HQ) in an alkaline medium (KOH 2.0 x 10(-2) mol/l) as fluorescent agent, is described. The fluorescence intensity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service