M5852
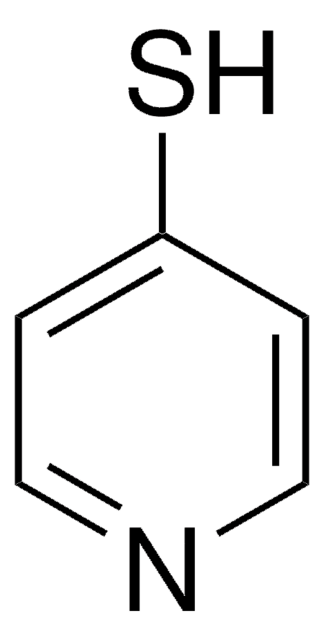
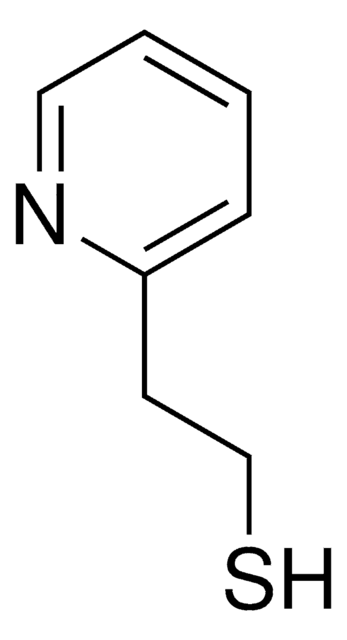
2-Mercaptopyridine
ReagentPlus®, 99%
Synonym(s):
2-Pyridinethiol, 2-Pyridyl mercaptan
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C5H5NS
CAS Number:
Molecular Weight:
111.16
Beilstein/REAXYS Number:
105787
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product line
ReagentPlus®
assay
99%
mp
127-130 °C (lit.)
storage temp.
2-8°C
SMILES string
Sc1ccccn1
InChI
1S/C5H5NS/c7-5-3-1-2-4-6-5/h1-4H,(H,6,7)
InChI key
WHMDPDGBKYUEMW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Mercaptopyridine is an organosulfur compound that contains more than one hetero atom. It is commonly used as a nucleophile in various organic synthesis reactions and plays important role in coordination chemistry as a versatile ligand due to its π-acidic nature.
Application
Employed as a ligand in metal complexes.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2, 4-bis (bromomethyl)-1, 3, 5-trimethylbenzene with 2-mercaptopyridine based derivative: Synthesis, crystal structure, in vitro anticancer activity, DFT, Hirshfeld surface analysis, antioxidant, DNA binding and molecular docking studies
Radhakrishnan Nandini A, et al.
Journal of Molecular Structure, 1251, 131981-131981 (2022)
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 3, 741-741 (1992)
Étienne Rochette et al.
Journal of the American Chemical Society, 141(31), 12305-12311 (2019-07-10)
The potential advantages of using arylboronic esters as boron sources in C-H borylation are discussed. The concept is showcased using commercially available 2-mercaptopyridine as a metal-free catalyst for the transfer borylation of heteroarenes using arylboronates as borylation agents. The catalysis
Inorganic Chemistry, 33, 5167-5167 (1994)
Elucidating the adsorption of 2-Mercaptopyridine drug on the aluminum phosphide (Al12P12) nanocage: A DFT study
Al-shimaa R SM, et al.
Heliyon, 9 (2023)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service