P56100
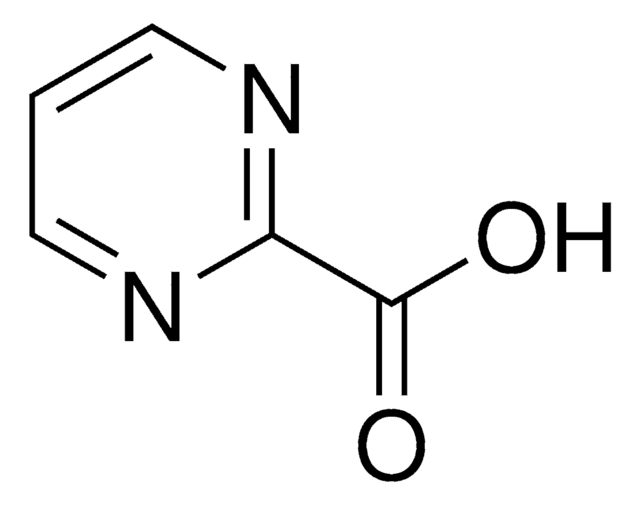
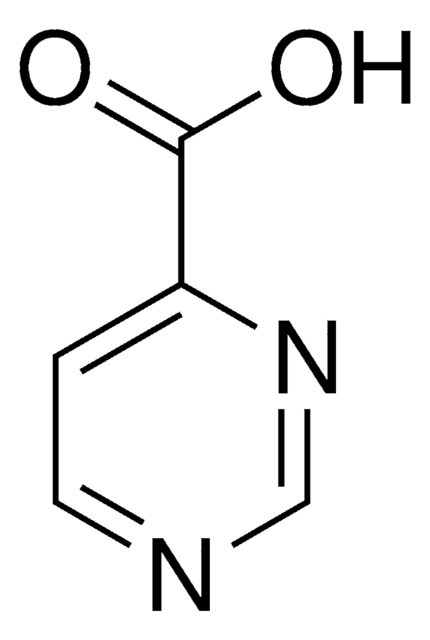
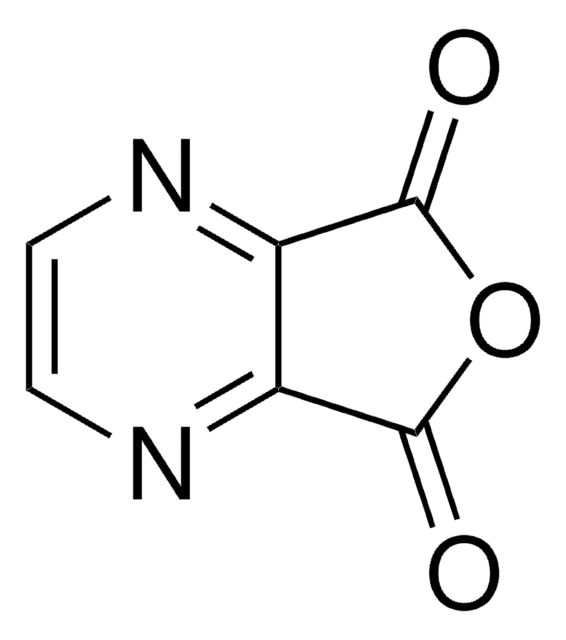
Pyrazinecarboxylic acid
99%
Synonym(s):
Pyrazinoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H4N2O2
CAS Number:
Molecular Weight:
124.10
Beilstein/REAXYS Number:
112305
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
powder
mp
222-225 °C (dec.) (lit.)
SMILES string
OC(=O)c1cnccn1
InChI
1S/C5H4N2O2/c8-5(9)4-3-6-1-2-7-4/h1-3H,(H,8,9)
Inchi Key
NIPZZXUFJPQHNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stefania Tanase et al.
Dalton transactions (Cambridge, England : 2003), (15)(15), 2026-2033 (2008-04-03)
The reactivity towards H(2)O(2) of the complexes [Fe(pca)(2)(py)(2)].py (1) and Na(2){[Fe(pca(3))](2)O}.2H(2)O.CH(3)CN (2) (where pca(-) is pyrazine-2-carboxylate) and their catalytic activity in the oxidation of hydrocarbons is reported. Addition of H(2)O(2) to 1 results in the formation of a dinuclear Fe(III)-(mu-O)-Fe(III)
Mirko Zimic et al.
Tuberculosis (Edinburgh, Scotland), 92(1), 84-91 (2011-10-19)
Pyrazinamide is one of the most important drugs in the treatment of latent Mycobacterium tuberculosis infection. The emergence of strains resistant to pyrazinamide represents an important public health problem, as both first- and second-line treatment regimens include pyrazinamide. The accepted
Ping Lu et al.
Antimicrobial agents and chemotherapy, 55(11), 5354-5357 (2011-08-31)
Pyrazinoic acid, the active form of the first-line antituberculosis drug pyrazinamide, decreased the proton motive force and respiratory ATP synthesis rates in subcellular mycobacterial membrane assays. Pyrazinoic acid also significantly lowered cellular ATP levels in Mycobacterium bovis BCG. These results
Rustam Z Khaliullin et al.
The journal of physical chemistry. B, 109(38), 17984-17992 (2006-07-21)
Experimental studies by Shul'pin and co-workers have shown that vanadate anions in combination with pyrazine-2-carboxylic acid (PCA identical with pcaH) produce an exceptionally active complex that promotes the oxidation of alkanes and other organic molecules. Reaction of this complex with
Mohamed Abdel-Aziz et al.
European journal of medicinal chemistry, 45(8), 3384-3388 (2010-05-22)
A series of pyrazine-2-carboxylic acid hydrazide derivatives were synthesized and screened for their activity against Mycobacterium tuberculosis. The results show that pyrazine-2-carboxylic acid hydrazide-hydrazone derivatives 3a-l were less active than pyrazinamide. In contrast, the N(4)-ethyl-N(1)-pyrazinoyl-thiosemicarbazide 4 showed the highest activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service