All Photos(1)
About This Item
Empirical Formula (Hill Notation):
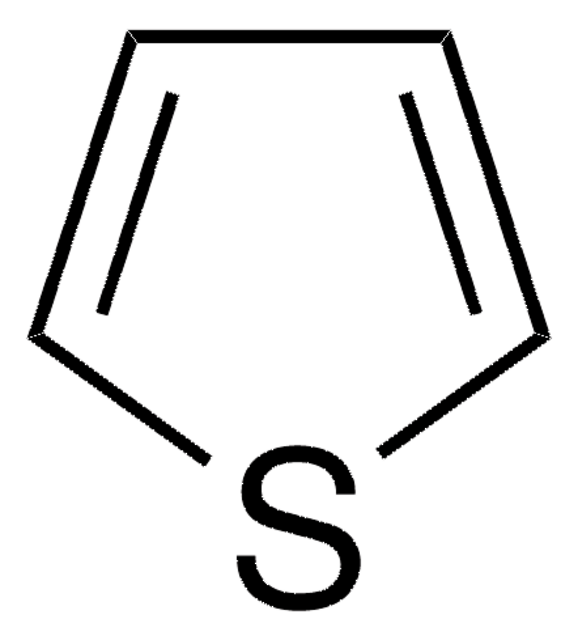
C4H4S
CAS Number:
Molecular Weight:
84.14
Beilstein/REAXYS Number:
103222
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.9 (vs air)
Quality Level
vapor pressure
40 mmHg ( 12.5 °C)
assay
≥99%
autoignition temp.
743 °F
expl. lim.
12.5 %
refractive index
n20/D 1.529 (lit.)
bp
84 °C (lit.)
mp
−38 °C (lit.)
density
1.051 g/mL at 25 °C (lit.)
SMILES string
c1ccsc1
InChI
1S/C4H4S/c1-2-4-5-3-1/h1-4H
InChI key
YTPLMLYBLZKORZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Thiophene is an essential precursor to synthesize a wide range of conjugated organosulfur moieties, sulfur-containing polycyclic aromatic hydrocarbons, macrocycles, and pharmaceutical compounds. It has been widely used in the synthesis of polythiophenes and a host of active materials for organic field effect transistors (OFETs) and organic solar cells (OSCs).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
15.8 °F
flash_point_c
-9 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solution-processed and high-performance organic solar cells using small molecules with a benzodithiophene unit.
Zhou J, et al.
Journal of the American Chemical Society, 135(23), 8484-8487 (2013)
Sukyoung Hwang et al.
Scientific reports, 5, 11201-11201 (2015-06-19)
The growth kinetics of polymer thin films prepared by plasma-based deposition method were explored using atomic force microscopy. The growth behavior of the first layer of the polythiophene somewhat differs from that of the other layers because the first layer
Thiophene-based push-pull chromophores for small molecule organic solar cells (SMOSCs)
Malytskyi V, et al.
Royal Society of Chemistry Advances, 5(1), 354-397 (2015)
Construction of synthetic macrocyclic compounds possessing subheterocyclic rings, specifically pyridine, furan, and thiophene.
Newkome GR, et al.
Chemical Reviews, 77(4), 513-597 (1977)
Thiophene Polymer Semiconductors for Organic Thin-Film Transistors
Ong BS, et al.
Chemistry?A European Journal , 14(16), 4766-4778 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service