D32202
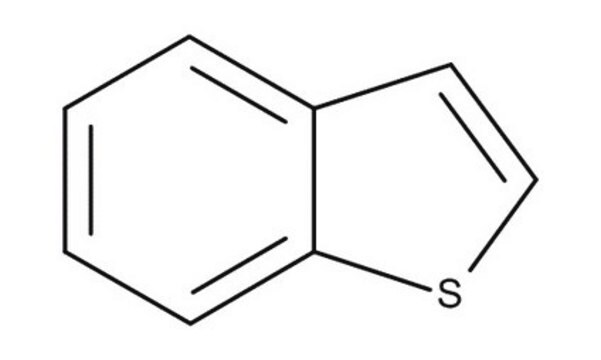
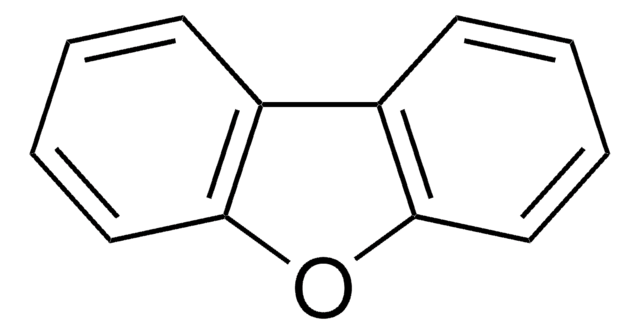
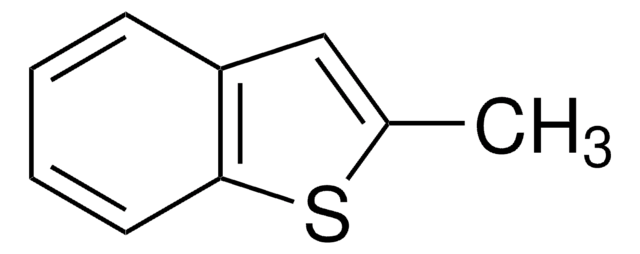
Dibenzothiophene
98%
Synonym(s):
DBT, Diphenylene sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H8S
CAS Number:
Molecular Weight:
184.26
Beilstein/REAXYS Number:
121101
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
powder or crystals
bp
332-333 °C (lit.)
mp
97-100 °C (lit.)
SMILES string
c1ccc2c(c1)sc3ccccc23
InChI
1S/C12H8S/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H
InChI key
IYYZUPMFVPLQIF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dibenzothiophene (DBT) can be used as:
- A starting material for the synthesis of corresponding sulfoxide and sulfone by oxidative desulfurization using various catalysts.
- A template for the synthesis of surface molecular imprinted polymer (SMIP). SMIP is applicable for the removal of dibenzothiophene during desulfurization of the gasoline
- A precursor for the synthesis of DBT based π-conjugating polymers.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
338.0 °F
flash_point_c
170 °C
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Olga Senko et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-08)
Sulfur recovery from organic molecules such as toxic sulfones is an actual problem, and its solution through the use of environmentally friendly and nature-like processes looks attractive for research and application. For the first time, the possible bioconversion of organic
Oxidative desulfurization of dibenzothiophene and diesel over [Bmim]3PMo12O40
Zhang J, et al.
J. Catal., 279(2), 269-275 (2011)
Hydrodesulfurization of dibenzothiophene and 4, 6-dimethyldibenzothiophene over sulfided NiMo/γ-Al2O3, CoMo/γ-Al2O3, and Mo/γ-Al2O3 catalysts
Egorova, Marina and Prins, Roel
J. Catal., 225(2), 417-427 (2004)
Synthesis of novel π-conjugating polymers based on dibenzothiophene
Nemoto N, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 41(10), 1521-1526 (2003)
Synthesis and characterization of a surface molecular imprinted polymer as a new adsorbent for the removal of dibenzothiophene
Yang W, et al.
Journal of Chemical and Engineering Data, 57(6), 1713-1720 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service