H-902
α-Hydroxymidazolam solution
100 μg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
100 μg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
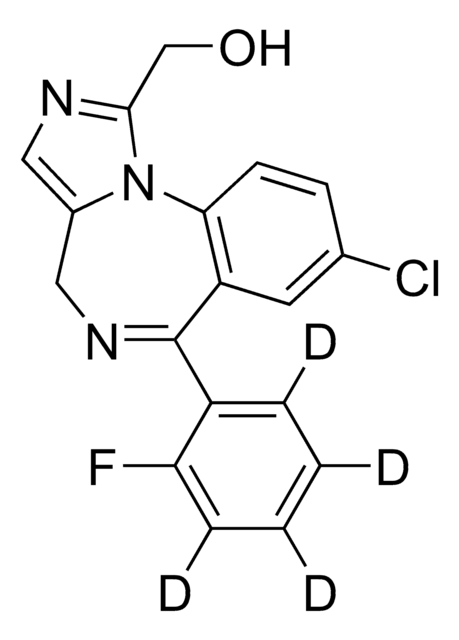
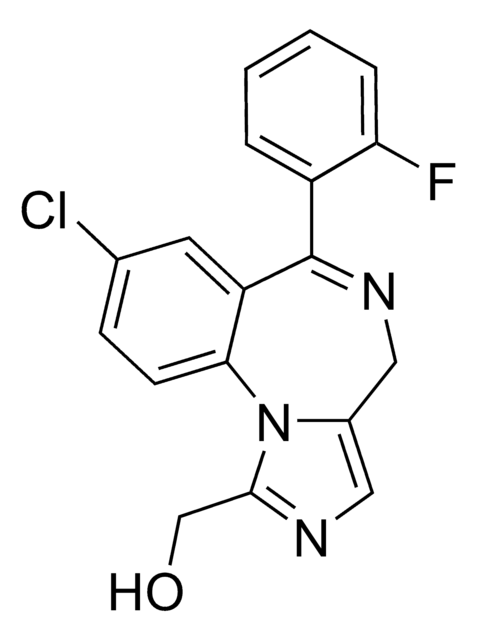
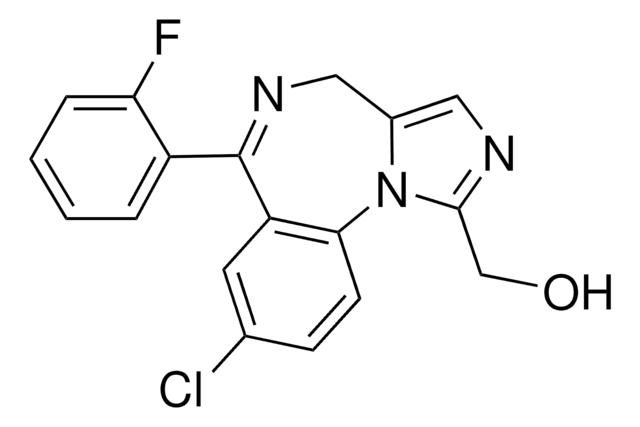
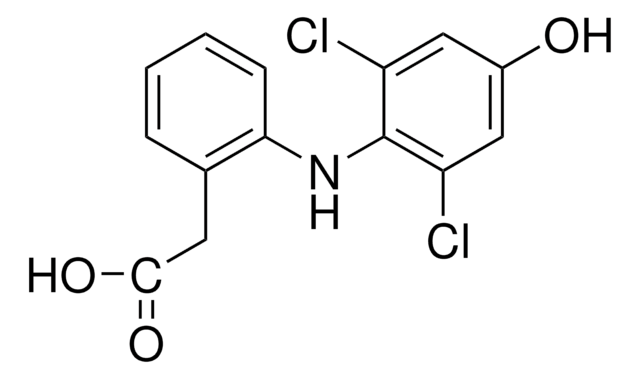
OCc1ncc2CN=C(c3ccccc3F)c4cc(Cl)ccc4-n12
InChI
1S/C18H13ClFN3O/c19-11-5-6-16-14(7-11)18(13-3-1-2-4-15(13)20)22-9-12-8-21-17(10-24)23(12)16/h1-8,24H,9-10H2
InChI key
QHSMEGADRFZVNE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service