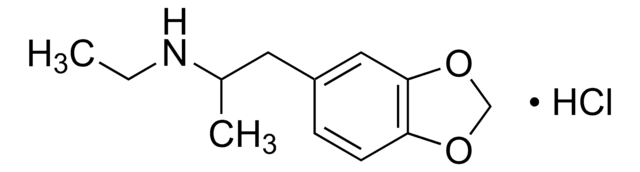
M-013
(±)-MDMA solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
(±)-3,4-Methylenedioxymethamphetamine
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule D (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIA (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
−20°C
SMILES string
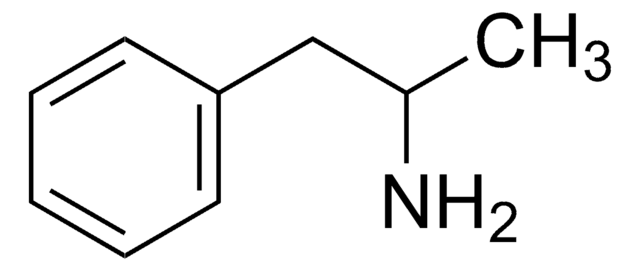
CC(CC1=CC=C(OCO2)C2=C1)NC
InChI
1S/C11H15NO2/c1-8(12-2)5-9-3-4-10-11(6-9)14-7-13-10/h3-4,6,8,12H,5,7H2,1-2H3
InChI key
SHXWCVYOXRDMCX-UHFFFAOYSA-N
General description
(±)-3,4-Methylenedioxymethamphetamine (MDMA) belongs to the class of racemic amphetamine-type stimulant drugs. It has psychoactive properties and is an active ingredient of the synthetic hallucinogenic drug, ecstasy. It is an illicit drug known by a variety of street names, including "Ecstasy," "E," "X," and "XTC". An analog of the phenethylamine class, MDMA is abused for its euphoric and psychedelic effects.
Application
- Electrochemical detection of morphine and MDMA from human serum and urine samples using a sensitive sensor based on platinum nanoparticles deposited on carbon nanohorns (CNHs)
- Enantiomeric assessment of seized drugs of amphetamine, methamphetamine, and MDMA by two methods of liquid chromatography coupled with tandem mass spectrometry (MS/MS) and diode array detector (DAD)
- Development and validation of a stereoselective liquid chromatography-tandem mass spectrometric (LC-MS/MS) procedure for the analysis of MDMA and its metabolites in human plasma samples
- Matrix-assisted laser desorption/ionization triple quadrupole tandem mass spectrometric (MALDI-QqQ-MS/MS) based analysis of MDMA and its metabolite 3,4-methylenedioxyamphetamine in oral fluid samples
- Differential pulse voltametric (DPV) identification and quantification of MDMA in seized drug samples using boron-doped diamond electrode (BDDE)
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Recommended products
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service