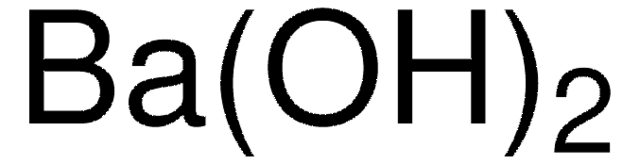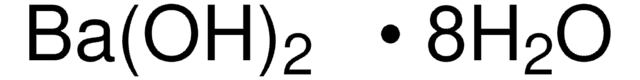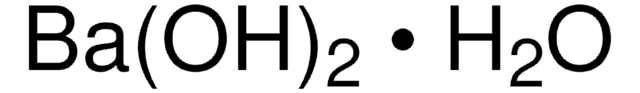1.01737
Barium hydroxide octahydrate
for analysis EMSURE® ACS,ISO,Reag. Ph Eur
Synonym(s):
Barium hydroxide octahydrate, Caustic baryta, Barium oxide hydrate octahydrate
Select a Size
Select a Size
About This Item
Recommended Products
grade
ACS reagent
Quality Level
agency
reag. ISO
reag. Ph. Eur.
product line
EMSURE®
assay
≥98.0% (acidimetric)
form
solid
potency
550 mg/kg LD50, oral (Rat)
impurities
≤0.005% Substances insoluble in dilute hydrochloric acid
pH
14 (20 °C in H2O, saturated aqueous solution)
mp
78 °C
Related Categories
Application
- Determination of phosphorylated and O-glycosylated sites by chemical targeting (CTID) at ambient temperature.: This study explores the use of chemical targeting to identify phosphorylated and O-glycosylated sites, essential for understanding protein modifications. Barium hydroxide is utilized for its effectiveness in ambient temperature conditions, contributing to more efficient analytical methods (Hathaway, 2007).
- Wet-chemical synthesis of crystalline BaTiO3 from stable chelated titanium complex: formation mechanism and dispersibility in organic solvents.: The research demonstrates the wet-chemical synthesis of BaTiO3, highlighting the formation mechanism and its dispersibility. Barium hydroxide plays a crucial role in stabilizing the chelated titanium complex, making it a valuable component in material synthesis and analytical chemistry (Pramanik et al., 2006).
- Synthesis and analgesic effect of normorphine-3- and -6-glucuronides.: This paper details the synthesis of normorphine glucuronides, where barium hydroxide is used in the synthetic process. The study contributes to the understanding of analgesic properties and the chemical synthesis of pharmacologically relevant compounds (Oguri et al., 1989).
Analysis Note
Substances insoluble in dilute hydrochloric acid: ≤ 0.005 %
Carbonate (as BaCO₃): ≤ 2.0 %
Chloride (Cl): ≤ 0.001 %
Sulphide (S): ≤ 0.0005 %
Heavy metals (as Pb): ≤ 0.0005 %
Ca (Calcium): ≤ 0.002 %
Fe (Iron): ≤ 0.0005 %
K (Potassium): ≤ 0.01 %
Na (Sodium): ≤ 0.01 %
Pb (Lead): ≤ 0.001 %
Sr (Strontium): ≤ 0.5 %
Corresponds to ACS,ISO,Reag. Ph Eur
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service