5.04537
PKM2 Activator III
Synonym(s):
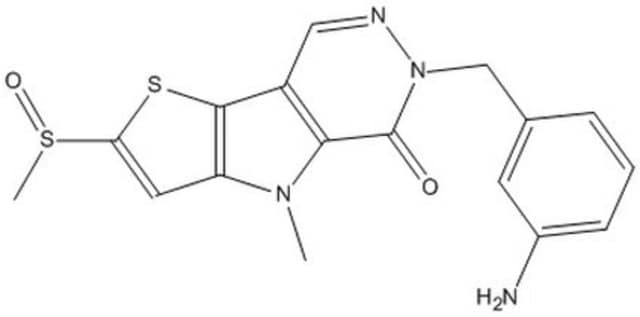
PKM2 Activator III, Pyruvate Kinase M2 Activator III, N-(4-(4-(2-Methoxyphenyl)piperazine-1-carbonyl)phenyl)quinoline-8-sulfonamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C27H26N4O4S
CAS Number:
Molecular Weight:
502.58
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
assay
≥99% (HPLC)
Quality Level
form
powder
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
off-white
solubility
DMSO: 5 mg/mL
storage temp.
2-8°C
General description
A cell-permeable quinoline-sulfonamide that acts as a potent allosteric PKM2 activator both in cell-free enzymatic assays (AC50 = 17 nM with 40 ng PKM2/200 µL) and in cultures (AC50 = 45 nM in A549 cells) via a high affinity, 2:1 stoichiometric binding, effectively locking PKM2 in an active tetrameric state resistant to known intracellular negative regulators of FBP-activated PKM2 tetramer. PKM2 stimulation by compound treatment is shown to result in decreased serine biosynthesis (by 56%; 500 nM for 24 h) with concomitant increase in serine influx as a compensating mechanism for maintaining cellular serine level necessary for supporting A549 proliferation. Simultaneous PKM2 activation and culture serine withdrawal results in cytostatic A549 growth arrest, but not apoptosis.
A cell-permeable quinoline-sulfonamide that acts as a potent allosteric PKM2 activator both in cell-free enzymatic assays (AC50 = 17 nM with 40 ng recombinant PKM2/200 µL) and in cultures (AC50 = 45 nM in A549 cells) via a high affinity (no dissociation in >1.5 h), 2:1 (compound to PKM2 tetramer) stoichiometric binding, effectively locking PKM2 in an active tetrameric state that is resistant to known intracellular negative regulators of PKM2 tetramer induced by the natural activator FBP (fructose 1,6-bisphosphate). PKM2 stimulation by compound treatment is shown to result in decreased serine biosynthesis (by 56%; 500 nM for 24 h) with concomitant increase in serine influx as a compensating mechanism for maintaining cellular serine level necessary for supporting A549 proliferation. Simultaneous PKM2 activation by drug treatment and culture serine withdrawal results in depletion of cellular serine pool (by ~70% in 24 h) and induction of cytostatic A549 growth arrest (by 56%; 72 h 1 µM drug treatment in BME + NEAA - Ser), but not apoptosis. Serine-dependent proliferation inhibition upon PKM2 activation is reported to be cell-specific, while it is observed in A549 and H460 cultures, SW480 and H522 are not affected.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
PKM2
PKM2
Reversible: no
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Kung, C., et al. 2012. Chem. Biol.19, 1187.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service