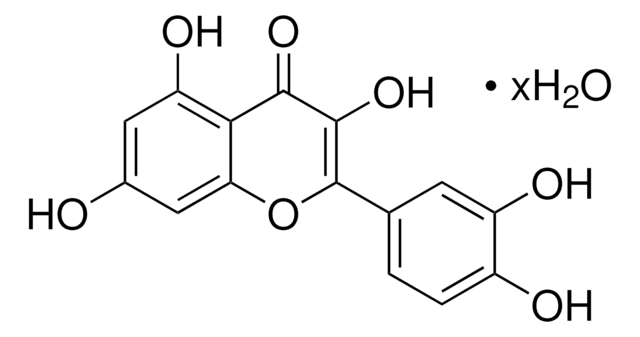
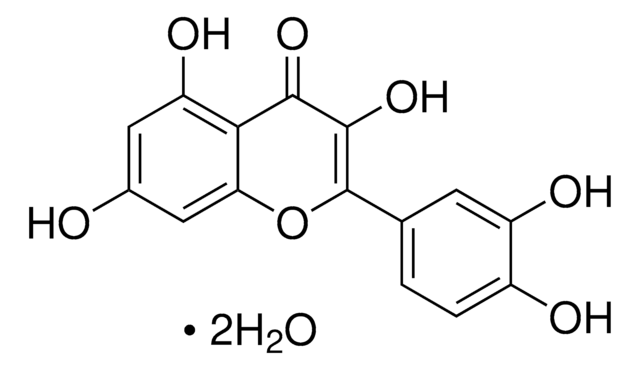
Q4951
Quercetin
≥95% (HPLC), solid, flavonoid antioxidant
Synonym(s):
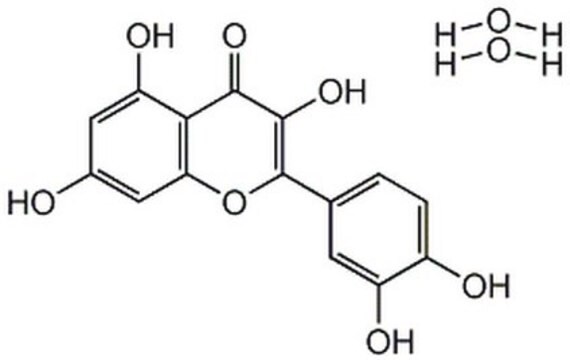
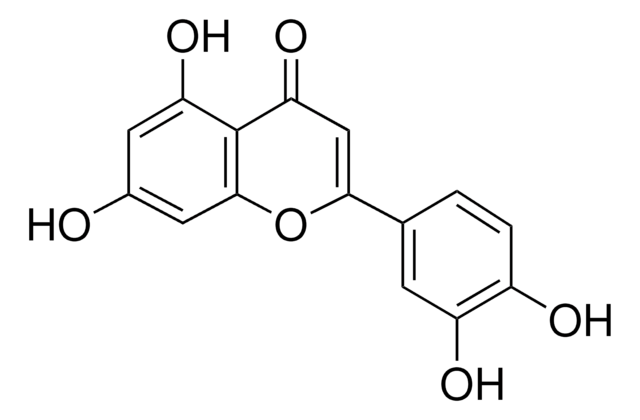
2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, 3,3′,4′,5,6-Pentahydroxyflavone
About This Item
Recommended Products
Product Name
Quercetin, ≥95% (HPLC), solid
biological source
synthetic (organic)
assay
≥95% (HPLC)
form
solid
mp
316.5 °C
solubility
water: practically insoluble
storage temp.
room temp
SMILES string
OC1=CC(O)=C2C(OC(C3=CC=C(O)C(O)=C3)=C(O)C2=O)=C1
InChI
1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H
InChI key
REFJWTPEDVJJIY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Quercetin has been used as an antioxidant which reversed the immunosuppressive effects of high glucose and hyperglycemic sera in type 2 diabetic patients.[3]
- It has been used as a detoxifying phytochemical in Apis mellifera.[4]
- It has been used as a positive control in DPPH (2,2- diphenyl-1-picryhydrazyl) radical scavenging assay. It has also been used for the preparation of calibration curve to determine total flavonoid content.[5]
Biochem/physiol Actions
Features and Benefits
Preparation Note
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for Kinase Phosphatase Biology
Discover Bioactive Small Molecules for Lipid Signaling Research
Protocols
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service