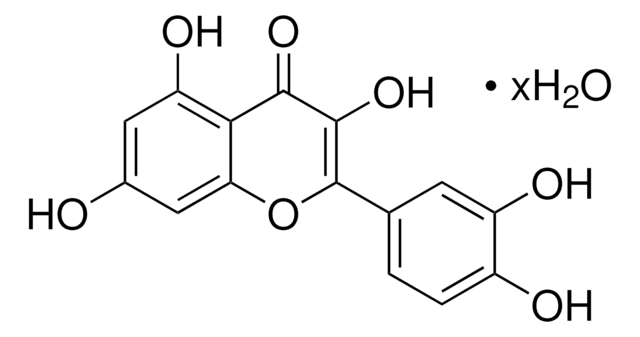
L9283
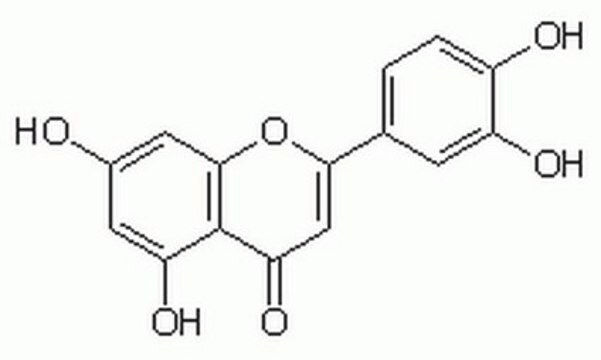
Luteolin
≥98% (TLC), powder, antioxidant
Synonym(s):
3′,4′,5,7-Tetrahydroxyflavone
About This Item
Recommended Products
Product Name
Luteolin, ≥98% (TLC), powder
Quality Level
assay
≥98% (TLC)
form
powder
shelf life
3 yr
color
yellow
mp
~330 °C (lit.)
storage temp.
2-8°C
SMILES string
Oc1cc(O)c2C(=O)C=C(Oc2c1)c3ccc(O)c(O)c3
InChI
1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to induce and elucidate the apoptotic pathway in renal cell carcinoma 786-O cells[1]
- as an additive in M9 minimal medium to induce nodF gene expression[6]
- as a reference standard to qualitatively and quantitatively analyse luteolin using reverse phase-high performance liquid chromatography with diode array detector (RP-HPLC-DAD)[2]
- as a reaction supplement for β-galactosidase assay[7]
- to elucidate the anti-inflammatory efficacy of luteolin in pseudorabies virus infected RAW264.7 cell line by measuring the anti-inflammatory mediators production and also cell viability and cytotoxicity assay[4]
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service