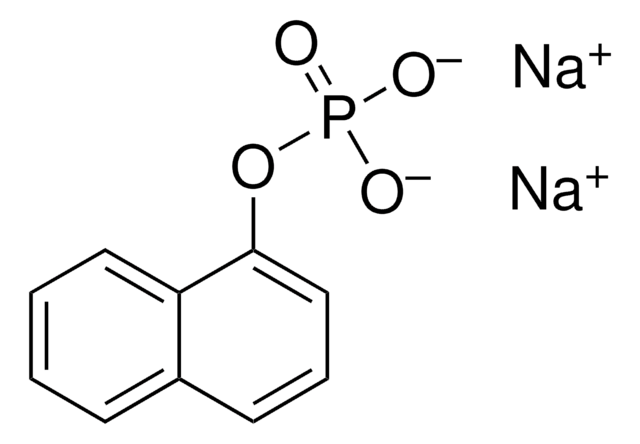
10108227001
Roche
Acid Phosphatase
grade II, from potato
Synonym(s):
phosphatase, acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
form
lyophilized
specific activity
~2 units/mg protein (at 25 °C with 4-nitrophenyl phosphate as the substrate)
packaging
pkg of 500 mg
manufacturer/tradename
Roche
optimum pH
4.9-5.6
shipped in
wet ice
Related Categories
General description
Orthophosphoric-monoester phosphohydrolase (acid optimum).
Acid phosphatase is a non-specific phosphomonoesterase, found in the cytoplasmic and cell wall sections of swelling potato tubers. It possesses several acidic amino acid residues. It is found to be present in up to six isoforms with varying degree of glycosylation. The major potato isoform is a homodimer having a molar mass of 96kDa.
Acid phosphatase is a non-specific phosphomonoesterase, found in the cytoplasmic and cell wall sections of swelling potato tubers. It possesses several acidic amino acid residues. It is found to be present in up to six isoforms with varying degree of glycosylation. The major potato isoform is a homodimer having a molar mass of 96kDa.
Biochem/physiol Actions
Acid phosphatase maintains the concentration of inorganic phosphate. It hydrolyzes orthophosphate monoesters under acidic conditions and also takes part in tuber development in potatoes. It eliminates phosphate groups from phosphoproteins, such as casein, riboflavin binding protein, pepsinogen, ovalbumin, and phosvitin.
Storage and Stability
Store at 2 to 8 °C. (Store dry!)
Other Notes
For life science research only. Not for use in diagnostic procedures.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
does not flash
flash_point_c
does not flash
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Acid phosphatases
H Bull
Journal of Clinical Pathology, 55, 65-72 (2002)
K. S. Gellatly et al.
Plant physiology, 106(1), 223-232 (1994-09-01)
The major acid phosphatase (APase) from potato (Solanum tuberosom L. cv Chiefton) tubers has been purified 2289-fold to near homogeneity and a final O-phospho-L-tyrosine (P-Tyr) hydrolyzing specific activity of 1917 [mu]mol Pi produced min-1 mg-1 of protein. Nondenaturing polyacrylamide gel
Da-Yong Wang et al.
Journal of integrative plant biology, 50(6), 733-741 (2008-08-21)
APase activity is involved in regulating many physiological and developmental events by affecting the resorption process. In this study, we investigate the role of APase activity in tuber development in potato. APase activities were mainly localized in cytoplasm, gaps among
Sanaullah Khan et al.
Natural product research, 30(5), 570-573 (2015-04-19)
Acid phosphatase-I (Apase-I) from seeds of Nelumbo nucifera was purified to electrophoretic homogeneity by combination of ammonium sulfate precipitation, size-exclusion and ion exchange chromatography. SDS-PAGE of purified Apase-I gave a single band with molecular mass of 80 kDa under reducing
E W Bingham et al.
Biochimica et biophysica acta, 429(2), 448-460 (1976-04-08)
Potato acid phosphatase (EC 3.1.3.2) was used to remove the eight phosphate groups from alphas1-casein. Unlike most acid phosphatases, which are active at pH 6.0 or below, potato acid phosphatase can catalyze the dephosphorylation of alphas1-casein at pH 7.0. Although
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service