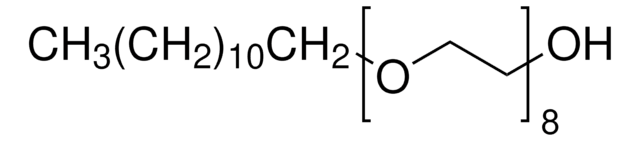10634425001
Roche
n-Octylglucoside
non-ionic
Synonym(s):
Octyl β-D-glucopyranoside, n-Octyl glucoside, OGP
About This Item
Recommended Products
assay
99% (GC)
mol wt
micellar avg mol wt 25,000
292.4
packaging
pkg of 10 g
manufacturer/tradename
Roche
aggregation number
84
technique(s)
dialysis: suitable
CMC
20-25 mM (20-25°C)
transition temp
cloud point >100 °C
shipped in
ambient
SMILES string
CCCCCCCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O
InChI
1S/C14H28O6/c1-2-3-4-5-6-7-8-19-14-13(18)12(17)11(16)10(9-15)20-14/h10-18H,2-9H2,1H3/t10-,11-,12+,13-,14-/m1/s1
InChI key
HEGSGKPQLMEBJL-RKQHYHRCSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Quality
Ease of removal*: ++
CMC**: 14.5 mM at +25°C
Formula: C14H28O6
Preparation Note
Working solution: Solubility: > 50% (w/v) in double-dist. water, Tris-HCl 0.05 mol/l; pH7.4 or K-phosphate buffer 0.1 mol/l, pH 7.0 at 25 °C.
Storage conditions (working solution): Stability in Solution
A stock solution is stable at 2 to 8 °C for approx. 3 days. We would expect a long term stability of 6-12 months if stored frozen in portions at -15 to -25 °C, provided bacterial contamination is avoided.
Storage and Stability
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service