08012
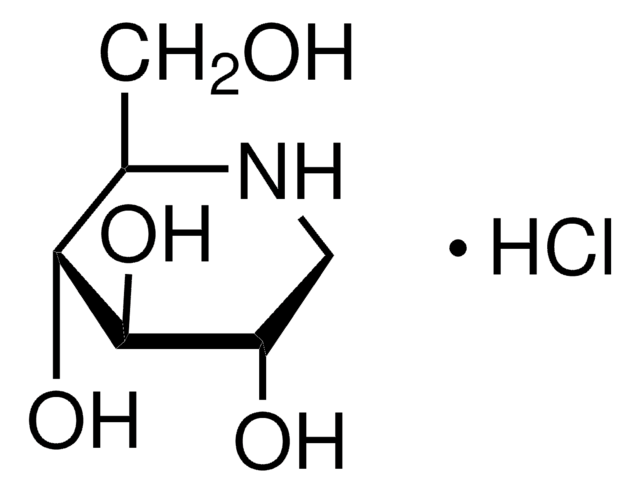
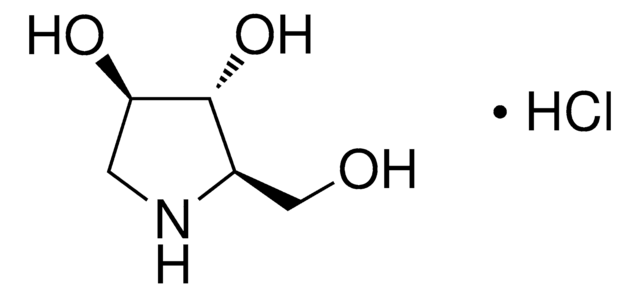
1-Deoxynojirimycin
analytical standard
Synonym(s):
1,5-Dideoxy-1,5-imino-D-sorbitol
About This Item
Recommended Products
grade
analytical standard
Quality Level
assay
≥95.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
format
neat
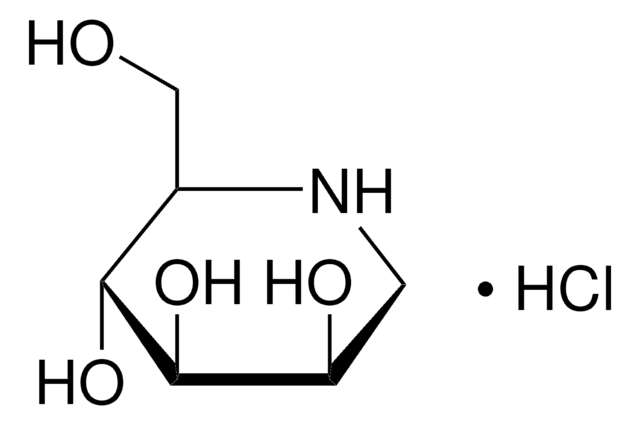
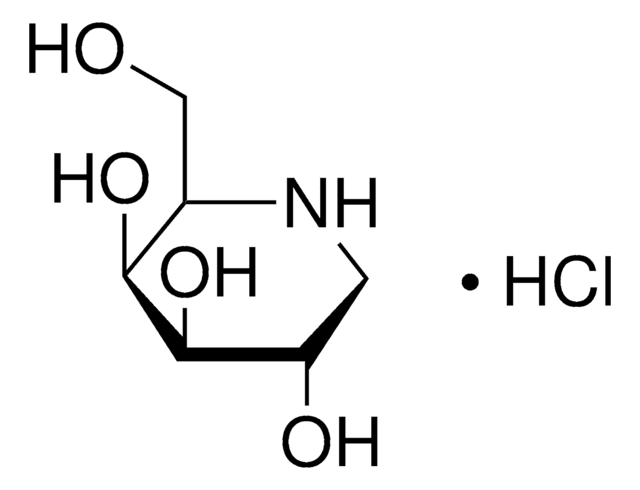
SMILES string
OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1
Inchi Key
LXBIFEVIBLOUGU-JGWLITMVSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
[1]
Application
- Mulberry leaves by high-performance liquid chromatography (HPLC) equipped with evaporative light scattering detector (ELSD)[2] as well as direct analysis in real-time (DART) ionization source coupled with triple quadrupole tandem mass spectrometry (MS/MS).[3]
- Silkworms by hydrophilic interaction chromatography (HILIC) with MS/MS operating on multiple reaction monitoring (MRM) mode.[4]
- Mulberry leaves of 132 varieties belonging to nine Morus species following its derivatization with fluorenylmethyl chloroformate (FMOC-Cl) by high-performance liquid chromatography (HPLC) combined with photodiode array (PDA) detector
- Development of an analytical method based on hydrophilic interaction chromatography (HILIC) coupled with tandem mass spectrometry (MS/MS) in positive ion electrospray ionization mode in the leaves of 32 cultivars of different Morus species
- HPLC method-based separation and quantification in the ethanol extracts of the leaves of Morus alba L. and Morus nigra with fluorimetric detection following pre-column derivatization with 9-fluorenylmethyl chloroformate
- 146 varieties of mulberry fruits from nine genera by HPLC in combination with UV-Vis dual-wavelength detection
- Human plasma by hybrid quadrupole/linear ion trap tandem mass spectrometry (QTRAP MS/MS).[5]
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service