PHR1909
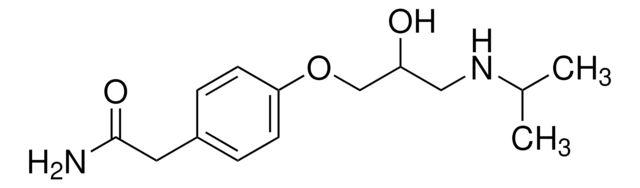
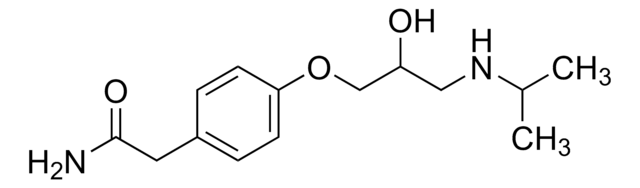
Atenolol
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Atenolol, (±)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide, 4-[2′-Hydroxy-3′-(isopropylamino)propoxy]phenylacetamide
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to BP 492
traceable to Ph. Eur. A1340000
traceable to USP 1044403
API family
atenolol
form
powder
CofA
current certificate can be downloaded
packaging
pkg of 500 mg
application(s)
pharmaceutical
storage temp.
2-30°C
SMILES string
CC(C)NCC(O)COc1ccc(CC(N)=O)cc1
InChI
1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)
InChI key
METKIMKYRPQLGS-UHFFFAOYSA-N
Gene Information
human ... ADRB1(153)
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Atenolol is a beta-1 blocker used in the treatment of various cardiovascular diseases.
Application
- Simultaneous analysis of atenolol and nifedipine using magnesium oxide-nanoplatelets (MgO-NPLs) modified screen-printed electrodes (SPEs) by differential pulse voltammetry (DPV) from pharmaceutical dosage forms and urine samples
- Voltammetric determination of amlodipine besylate and atenolol in their combined pharmaceutical formulations using a cathodically pretreated-boron doped diamond electrode (CP-BDDE)
- Spectrofluorimetric determination of atenolol using gold nanoparticles (AuNPs) based photo probes in commercial tablets
- Amperometric estimation of atenolol using bismuth vanadate-bismuth(III) oxide (BiVO4–Bi2O) composite electrode in pharmaceutical formulations and human urine samples
- Development of a reversed phase-high performance liquid chromatography (RP-HPLC) method coupled to a photodiode array detector (PDA) for the quantitative analysis of atenolol and trimetazidine in human urine and pharmaceutical tablets
Analysis Note
Footnote
Recommended products
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
TLC plates, with silica gel, gypsum as binder, International Pharmacopoeia compliant, for identification of Atenolol and Chlorthalidone in tablets.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service