T1807
L-(+)-Tartaric acid
BioXtra
Synonym(s):
(2R,3R)-(+)-Tartaric acid, L-Threaric acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(4)
Select a Size
Change View
About This Item
Linear Formula:
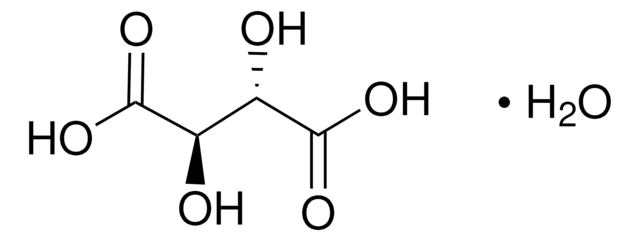
HO2CCH(OH)CH(OH)CO2H
CAS Number:
Molecular Weight:
150.09
Beilstein/REAXYS Number:
1725147
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
vapor density
5.18 (vs air)
Quality Level
product line
BioXtra
assay
≥98%
form
powder
autoignition temp.
797 °F
impurities
≤0.002% Phosphorus (P)
≤0.1% Insoluble matter
ign. residue
≤0.1%
mp
170-172 °C (lit.)
solubility
H2O: 1 M at 20 °C, clear, colorless
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tartaric acid is a dicarboxylic acid and occurs as two enantiomers and an achiral meso compound. The L-(+)-tartaric acid is extensively found in nature. It is an acid present in fruits such as grapes and bananas. It possesses an astringent and citrus flavor.
Application
L-(+)-Tartaric acid has been used:
- in filter-sterilized synthetic grape juice for feast fermentation
- in crystallization experiment
- to test the influence of stereospecific interactions between stereoisomers of tartaric acid and proteins during crystallogenesis
Biochem/physiol Actions
L-(+)-Tartaric acid serves as a donor ligand for biological processes. It is used as a food additive in candies and soft drinks to impart a sour taste.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
302.0 °F - closed cup
flash_point_c
150 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Higher Denticity Ligands
Comprehensive Coordination Chemistry II (2003)
4.16-human-environment interactions?Taste
Izawa K, et al.
Comprehensive Natural Products II, 631-671 (2010)
Acetogenin (Polypriopionate) Derived Auxiliaries: Tartaric Acid
Comprehensive Chirality (2012)
Effects of protein purity and precipitant stereochemistry on the crystallization of thaumatin
Asherie N, et al.
Crystal Growth & Design, 8(12), 4200-4207 (2008)
Tartrate chirality determines thaumatin crystal habit
Asherie N, et al.
Crystal Growth & Design, 9(9), 4189-4198 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service