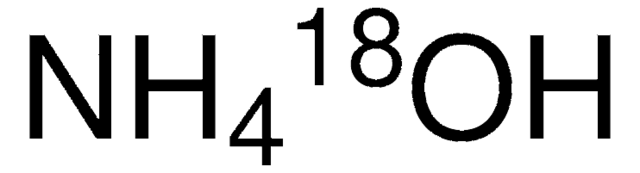30501
Ammonium hydroxide solution
puriss. p.a., reag. ISO, reag. Ph. Eur., ~25% NH3 basis
Synonym(s):
Ammonia aqueous, Ammonia water
About This Item
Recommended Products
agency
USP/NF
reag. ISO
reag. Ph. Eur.
description
related substances ≤ 2 ppm (as pyridine)
grade
puriss. p.a.
form
liquid
expl. lim.
27 %
concentration
~25% NH3
impurities
readily oxidisable substances, in accordance
≤0.0001% heavy metals (as Pb)
≤0.0005% KMnO4 red. matter (as O)
≤0.0018% non-volatile matter
evapn. residue
≤0.0018%
pH
11.7 (20 °C)
density
0.892-0.910 g/mL at 20 °C
0.9 g/mL at 25 °C (lit.)
anion traces
carbonate (CO32-): ≤60 ppm
carbonate (as CO2): ≤10 ppm
chloride (Cl-): ≤0.5 ppm
phosphate (PO43-): ≤0.5 ppm
silicate (as SiO2): ≤10 ppm
sulfate (SO42-): ≤2 ppm
sulfide (S2-): ≤0.2 ppm
cation traces
Ag: ≤0.02 ppm
Al: ≤0.5 ppm
Au: ≤0.1 ppm
Ba: ≤0.05 ppm
Bi: ≤0.1 ppm
Ca: ≤1 ppm
Cd: ≤0.05 ppm
Co: ≤0.05 ppm
Cr: ≤0.05 ppm
Cu: ≤0.1 ppm
Fe: ≤0.1 ppm
Ga: ≤0.02 ppm
In: ≤0.02 ppm
K: ≤0.5 ppm
Li: ≤0.02 ppm
Mg: ≤0.1 ppm
Mn: ≤0.05 ppm
Mo: ≤0.05 ppm
Na: ≤1 ppm
Ni: ≤0.05 ppm
Pb: ≤0.05 ppm
Pt: ≤0.1 ppm
Sn: ≤0.1 ppm
Sr: ≤0.2 ppm
Ti: ≤0.1 ppm
Tl: ≤0.05 ppm
Zn: ≤0.1 ppm
SMILES string
[NH4+].[OH-]
suitability
complies for appearance of solution
storage temp.
2-8°C
InChI
1S/H3N.H2O/h1H3;1H2
InChI key
VHUUQVKOLVNVRT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Smartphone-based digital image colorimetry for the determination of total capsaicinoid content in chili pepper extracts.: This study uses ammonium hydroxide solution in the extraction process, combined with smartphone-based colorimetric analysis, to accurately determine capsaicinoid levels, showcasing advancements in portable analytical techniques (Vakh C et al., 2024).
- Open acid dissolution-Ammonia solution extraction-ICP OES rapid determination of 7 trace metal elements in soil.: This research highlights the use of ammonium hydroxide solution for the efficient extraction of trace metals from soil, followed by ICP OES analysis, providing a rapid and accurate method for environmental monitoring (Wang J et al., 2023).
- Development and validation of a sensitive ultra-high-performance liquid chromatography-tandem mass spectrometry method for quantitative analysis of bambermycin in livestock and aquatic products: Implications for food safety control and regulatory enforcement.: Ammonium hydroxide solution is utilized in the sample preparation process for UHPLC-MS/MS analysis, ensuring accurate quantification of bambermycin in various food products (Park DH et al., 2023).
- Fluorescent Carbon Dots from Food Industry By-Products for Cell Imaging.: The study uses ammonium hydroxide solution in the synthesis of fluorescent carbon dots from food by-products, demonstrating a sustainable approach to producing imaging agents for biomedical applications (Mancini F et al., 2023).
- Constructing a heparin-modified penile decellularized scaffold to improve re-endothelialization in organizational reconstruction.: This research employs ammonium hydroxide solution in the decellularization process of tissue scaffolds, contributing to advancements in tissue engineering and regenerative medicine (Zhang H et al., 2022).
Packaging
Other Notes
The article number 30501-4X2.5L-M will be discontinued. Please order the single bottle 30501-2.5L-M which is physically identical with the same exact specifications.
The article number 30501-6X1L will be discontinued. Please order the single bottle 30501-1L which is physically identical with the same exact specifications.
The article number 30501-6X1L-M will be discontinued. Please order the single bottle 30501-1L-M which is physically identical with the same exact specifications.
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service