53747
Hyaluronic acid sodium salt from Streptococcus equi
bacterial glycosaminoglycan polysaccharide
Synonym(s):
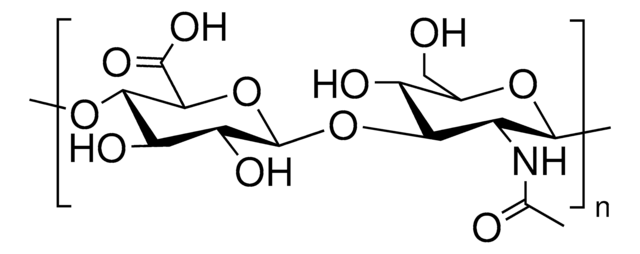
Poly(β-glucuronic acid-[1→3]-β-N-acetylglucosamine-[1→4]), alternating
About This Item
Recommended Products
biological source
(Streptococcus equi)
form
powder or crystals
mol wt
~1.5-1.8 x 10E6 Da
impurities
≤1% protein
color
white
solubility
H2O: 5 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
[Na+].CC(=O)N[C@@H]1C[C@H](O)[C@@H](CO)O[C@H]1O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2C([O-])=O
InChI
1S/C28H44N2O23.Na/c1-5(33)29-9-18(11(35)7(3-31)47-25(9)46)49-28-17(41)15(39)20(22(53-28)24(44)45)51-26-10(30-6(2)34)19(12(36)8(4-32)48-26)50-27-16(40)13(37)14(38)21(52-27)23(42)43;/h7-22,25-28,31-32,35-41,46H,3-4H2,1-2H3,(H,29,33)(H,30,34)(H,42,43)(H,44,45);/q;+1/t7-,8-,9-,10-,11-,12-,13+,14+,15-,16-,17-,18-,19-,20+,21+,22+,25-,26+,27-,28-;/m1./s1
InChI key
YWIVKILSMZOHHF-QJZPQSOGSA-N
General description
Application
- with methacrylic anhydride for synthesizing cross-linkable methacrylated HA hydrogel (Coll-MeHA)
- in phosphate buffer saline (PBS) to replace the PBS bath to vary the lubricant composition
- in the preparation of lubricant to study its effects on the boundary lubrication of human osteoarthritis (OA) cartilage
Biochem/physiol Actions
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Uncover more about glycosaminoglycans and proteoglycans including the structure of glycosaminoglycans (GAGs), the different types of GAGs, and their functions.
Glycosaminoglycans are large linear polysaccharides constructed of repeating disaccharide units.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service