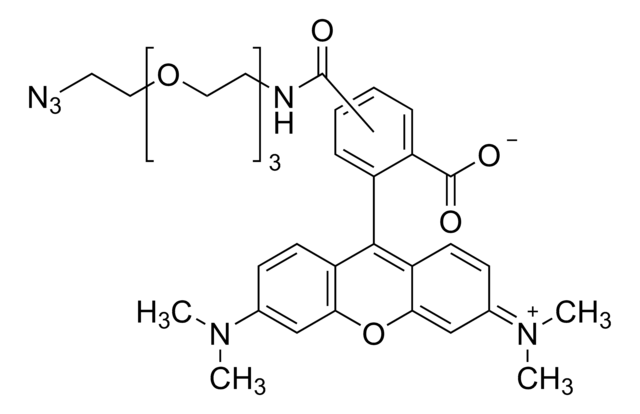
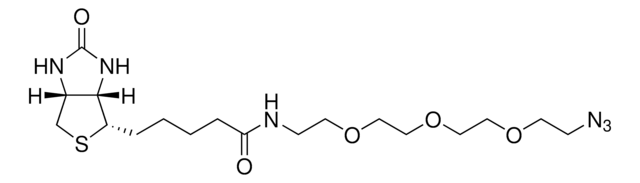
72709
Atto 488 azide
BioReagent, suitable for fluorescence, ≥90% (HPLC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352125
NACRES:
NA.32
Recommended Products
product line
BioReagent
Quality Level
assay
≥90% (HPLC)
form
solid
mol wt
Mw 903 g/mol
manufacturer/tradename
ATTO-TEC GmbH
λ
in methanol: water (1:1) (with 0.1% perchloric acid)
UV absorption
λ: 501-507 nm Amax
suitability
suitable for fluorescence
storage temp.
−20°C
General description
Atto 488 is a superior fluorescence label with high molecular absorption (90.000) and quantum yield (0.80) as well as sufficient stoke′s shift. It is optimised for excitation with argon laser, and is characterized by high photo stability.
The azide modification is suitable for reactions with alkyne groups (Huisgen reaction - "Click Chemistry").
The azide modification is suitable for reactions with alkyne groups (Huisgen reaction - "Click Chemistry").
Legal Information
This product is for Research use only. In case of intended commercialization, please contact the IP-holder (ATTO-TEC GmbH, Germany) for licensing.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Monitoring single membrane protein dynamics in a liposome manipulated in solution by the ABELtrap.
Rendler, T., et al.
arXiv, 1102-1102 (2011)
Analysis of fluorescent nanostructures in biological systems by means of spectral position determination microscopy (SPDM).
Muller, P., et al. et al.
Current Microscopy Contributions to Advances in Science and Technology, 1, 3-12 (2012)
Mohd A Mohd Ridzuan et al.
PloS one, 7(3), e33845-e33845 (2012-04-06)
An actomyosin motor complex assembled below the parasite's plasma membrane drives erythrocyte invasion by Plasmodium falciparum merozoites. The complex is comprised of several proteins including myosin (MyoA), myosin tail domain interacting protein (MTIP) and glideosome associated proteins (GAP) 45 and
Lucia De Rosa et al.
Organic & biomolecular chemistry, 10(2), 273-280 (2011-11-11)
In the last few years, the use of labeled proteins has significantly expanded in the life sciences. Now, labeled proteins are indispensable tools for a wide spectrum of biophysical and chemical biology applications. In particular, the quest for more sophisticated
Jonas K Hannestad et al.
ACS nano, 7(1), 308-315 (2012-12-12)
We use single-molecule fluorescence microscopy to monitor individual hybridization reactions between membrane-anchored DNA strands, occurring in nanofluidic lipid monolayer films deposited on Teflon AF substrates. The DNA molecules are labeled with different fluorescent dyes, which make it possible to simultaneously
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service