D7052
Dipeptidyl Peptidase IV from porcine kidney
lyophilized powder, ≥10 units/mg protein (Bradford)
Synonym(s):
Dipeptidyl aminopeptidase IV
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
Recommended Products
form
lyophilized powder
specific activity
≥10 units/mg protein (Bradford)
mol wt
124 kDa
packaging
vial of ≥0.75 unit
solubility
0.1 M Tris-HCl, pH 8.0: soluble 1 vial/mL, clear, colorless
UniProt accession no.
storage temp.
−20°C
Gene Information
pig ... DPPIV(397492)
Specificity
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z. Where X is a nonspecific residue at the N terminus and Z cannot be proline or hydroxyproline.
Application
Dipeptidyl peptidase IV from porcine kidney has been used in a study to investigate engraftment of donor cells following hepatocyte transplantation. Dipeptidyl peptidase IV from porcine kidney has also been used in a study to investigate the metabolism of glucagon-like peptide-2.
The enzyme from sigma has been used in the determination of DPP-IV inhibitory activity of peptides in porcine skin gelatin hydrolysates.
Biochem/physiol Actions
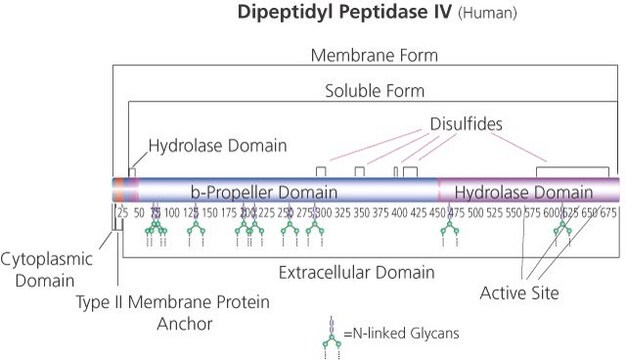
Native DPPIV is a ubiquitous type II transmembrane glycoprotein and a serine protease of the S9 prolyl-oligopeptidase family. In vivo, it is synthesized with a signal peptide, which functions as the membrane anchoring domain. There is an 88% sequence homology between the human and porcine kidney enzymes. Both exist as homodimers with a subunit molecular weight of ~30 kDa. The high mannose 100 kDa DPPIV precursor is processed in the Golgi to yield a 124 kDa heavily N-and O-linked mature glycoprotein. It is then sorted to the apical membrane through the concerted action of both N- and O-linked glycans and its association with lipid microdomains. The porcine enzyme contains 18.3% carbohydrates, which the glycan composition is 0.9% fucose, 3.4% mannose, 5.1% galactose, 8.2% glucosamine, and 0.7% sialic acid. DPPIV is highly expressed on endothelial cells, epithelial cells, and lymphocytes. It is also present in plasma in its soluble form.
The pH optimum for the protease is 7.8-8.0. Dipeptidyl peptidase does not require activators. It is inhibited by diisopropyl fluorophosphate, phenylmethanesulfonyl fluoride, and diethyl p-nitrophenyl phosphate.
Unit Definition
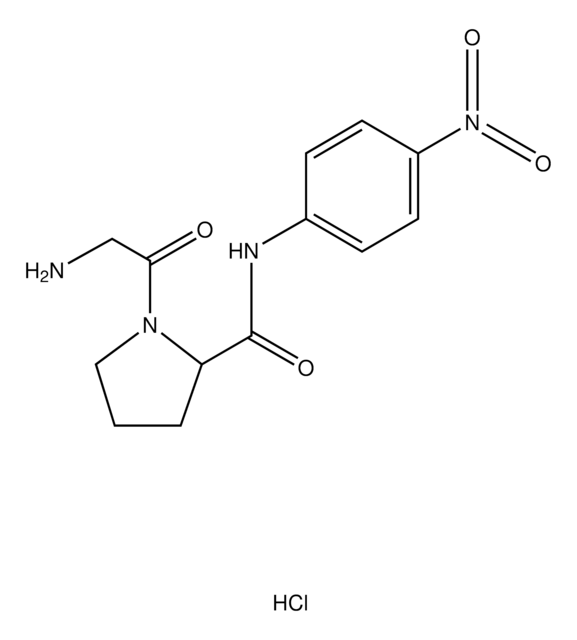
One unit will produce 1.0 μmole of p-nitroaniline from Gly-L-Pro p-nitroanilide per min in 100 mM Tris-HCl at pH 8.0 at 37 °C.
Physical form
Lyophilized powder containing Tris buffer salts
inhibitor
substrate
Product No.
Description
Pricing
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dipeptidyl Peptidase-IV Inhibitory Activity of Peptides in Porcine Skin Gelatin Hydrolysates.
Hsu, Kuo-Chiang, et al.
Bioactive Food Peptides in Health and Disease, 205-205 (2013)
Kosho Yamanouchi et al.
Hepatology (Baltimore, Md.), 49(1), 258-267 (2008-11-13)
Engraftment of donor hepatocytes is a critical step that determines the success of hepatocyte transplantation. Rapid and efficient integration of donor cells would enable prompt liver repopulation of these cells in response to selective proliferative stimuli offered by a preparative
A J Kenny et al.
The Biochemical journal, 157(1), 169-182 (1976-07-01)
Dipeptidyl peptidase IV, an enzyme that releases dipeptides from substrates with N-terminal sequences of the forms X-Pro-Y or X-Ala-Y, was purified 300-fold from pig kidney cortex. The kidney is the main source of the enzyme, where it is one of
K M Fukasawa et al.
Biochimica et biophysica acta, 657(1), 179-189 (1981-01-15)
Dipeptidyl peptidase IV (dipeptidylpeptide hydrolase, EC 3.4.14.-) has been purified from the microsomal fraction of pig liver, using an immunoaffinity chromatography, and its properties compared with those of the enzyme purified from pig kidney. The amino acid compositions of both
Thi Thu Huong Do et al.
American journal of physiology. Endocrinology and metabolism, 306(6), E668-E680 (2014-01-16)
The oligopeptide transporter peptide cotransporter-1 Slc15a1 (PEPT1) plays a major role in the regulation of nitrogen supply, since it is responsible for 70% of the dietary nitrogen absorption. Previous studies demonstrated that PEPT1 expression and function in jejunum are reduced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service