A8200
Aminopeptidase from Aeromonas proteolytica
lyophilized powder, 50-150 units/mg protein
Synonym(s):
AAP, Aminopeptidase from Vibrio proteolyticus, bacterial leucyl aminopeptidase
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
General description
A zinc-containing enzyme.
Specificity
Catalyzes the release of an N-terminal amino acid, preferentially leucine, but not glutamic or aspartic acids.
Application
Aminopeptidases are a family of widely distributed proteases, which may be used to study many significant biological processes such as protein maturation, hormone production, and peptide digestion. The enzyme has been used to measure the kinetic rate constant for the binding of bestatin, a general protease inhibitor, to aminopeptidase.
Biochem/physiol Actions
Aminopeptidase from Aeromonas proteolytica is a metalloenzyme, which contains 2 atoms of Zn2+ in a single polypeptide with an approximate molecular weight of 29.5 kDa as determined by sedimentation. This enzyme has a high degree of stability, being stable even at temperatures of 70 °C for several hours. Partial inactivation occurs in 8 M urea. Maximum stability and activity are between pH 8.0-8.5. Aminopeptidase from Aeromonas proteolytica can function as an esterase.
Aminopeptidase from Aeromonas proteolytica is involved in protein maturation, hormone production and peptide digestion.
Unit Definition
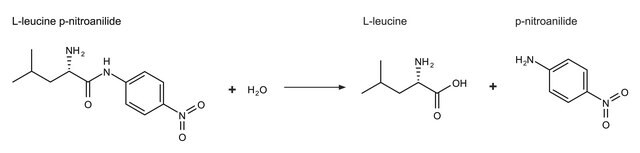
One unit will hydrolyze 1.0 μmole of L-leucine p-nitroanilide to L-leucine and p-nitroaniline per min at pH 8.0 at 25 °C.
Physical form
Lyophilized powder containing tricine buffer, pH 8.0, zinc chloride and stabilizer.
Preparation Note
Dissolves in water at 0.9-1.1 mg/mL concentration to form a clear, colorless solution.
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
James Kahn et al.
Biochemistry and molecular biology education : a bimonthly publication of the International Union of Biochemistry and Molecular Biology, 38(4), 238-241 (2011-05-14)
We have recently designed a biochemistry laboratory experiment for the purpose of providing students an advanced experience with enzyme kinetics and the kinetics of binding. Bestatin, a well-known and commercially available general protease inhibitor, is a slow-binding inhibitor of aminopeptidase
C Schalk et al.
Archives of biochemistry and biophysics, 294(1), 91-97 (1992-04-01)
The heat-stable aminopeptidase from Aeromonas proteolytica has been purified using two new procedures, with the aim of preparing large single crystals for X-ray analysis. In a first procedure, we tried to avoid any drastic conditions capable of inducing microheterogeneities in
William T Desmarais et al.
Structure (London, England : 1993), 10(8), 1063-1072 (2002-08-15)
The aminopeptidase from Aeromonas proteolytica (AAP) is a bridged bimetallic enzyme that removes the N-terminal amino acid from a peptide chain. To fully understand the metal roles in the reaction pathway of AAP we have solved the 1.20 A resolution
D Mahadevan et al.
Protein science : a publication of the Protein Society, 8(11), 2546-2549 (1999-12-14)
The Aeromonas proteolytica aminopeptidase (AMP), Pseudomonas sp. (RS-16) carboxypeptidase G2 (CPG2), and Streptomyces griseus aminopeptidase (SGAP) are zinc dependent proteolytic enzymes with cocatalytic zinc ion centers and a conserved aminopeptidase fold. A BLAST search with the sequence of the solved
Krzysztof P Bzymek et al.
The Journal of biological chemistry, 279(30), 31018-31025 (2004-05-13)
Glutamate 151 has been proposed to act as the general acid/base during the peptide hydrolysis reaction catalyzed by the co-catalytic metallohydrolase from Aeromonas proteolytica (AAP). However, to date, no direct evidence has been reported for the role of Glu-151 during
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service