C6019
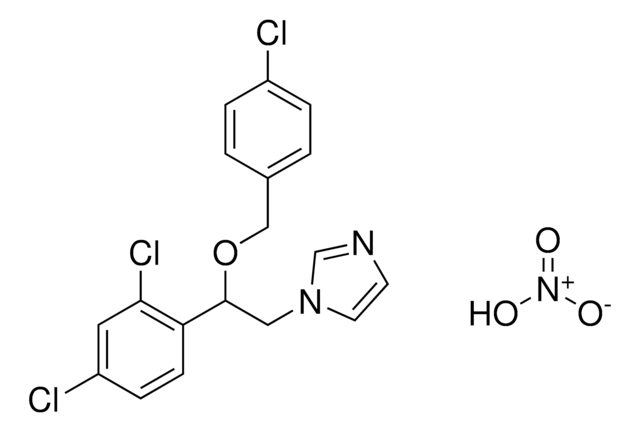
Clotrimazole
98.5-100.5% (dry basis), powder, Ca²⁺-activated K⁺ channels inhibitor
Synonym(s):
1-(o-Chloro-α,α-diphenylbenzyl)imidazole, 1-(o-Chlorotrityl)imidazole, 1-[(2-Chlorophenyl)diphenylmethyl]-1H-imidazole
About This Item
Recommended Products
product name
Clotrimazole,
form
powder
Quality Level
antibiotic activity spectrum
fungi
mode of action
cell membrane | interferes
protein synthesis | interferes
originator
Schering Plough
SMILES string
Clc1ccccc1C(c2ccccc2)(c3ccccc3)n4ccnc4
InChI
1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H
InChI key
VNFPBHJOKIVQEB-UHFFFAOYSA-N
Gene Information
human ... ABCB1(5243) , CYP17A1(1586) , CYP3A4(1576)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study the upregulation of the gene ERG11 that codes for an azole target enzyme lanosterol demethylase, in Candida species, upon treatment with azole antibiotics
- to study the development of resistance in Candida species isolated from patients undergoing prolonged antifungal treatment
- to induce stress granules via mitochondrial stress
- for the inhibition of in vitro formation of high density sickle cells induced by treatment with 1-chloro-2,4-dinitrobenzene (CDNB)
- to inhibit cytochrome P450 enzyme in cell cultures
Biochem/physiol Actions
Features and Benefits
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service