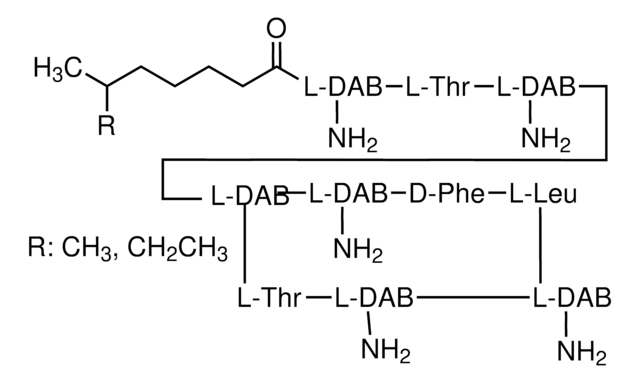
E4274
Endotoxin Removal solution
Remove lipopolysaccharides (LPS) from plasmid DNA preparations
Synonym(s):
ET removal buffer, ET removal reagent, ET removal solution, endo removal buffer, endo removal reagent, endo removal solution, endotoxin contamination removal, endotoxin removal buffer, endotoxin removal reagent, plasmid purification endo, plasmid purification endo free
About This Item
Recommended Products
Quality Level
Application
Biochem/physiol Actions
Quantity
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
FAQs on bacterial endotoxin contamination, details on endotoxin testing using the LAL assay, and tips to avoid contamination in cell cultures.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service