H9166
Vascular Endothelial Growth Factor 165 human
≥95% (SDS-PAGE), recombinant, expressed in HEK 293 cells, lyophilized powder, suitable for cell culture
Synonym(s):
Endothelial Growth Factor, Vascular Growth Factor
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Recommended Products
Product Name
Vascular Endothelial Growth Factor 165 human, VEGF165, recombinant, expressed in HEK 293 cells, HumanKine®, suitable for cell culture
biological source
human
Quality Level
recombinant
expressed in HEK 293 cells
assay
≥95% (SDS-PAGE)
form
lyophilized powder
potency
≤5 ng/mL EC50
quality
endotoxin tested
mol wt
dimer 45 kDa (glycosylated)
packaging
pkg of 5x10 μg
pkg of 10 μg
General description
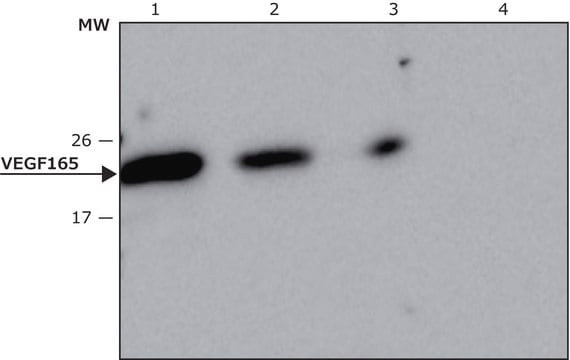
Vascular endothelial growth factor 121 (VEGF121) belongs to the PDGF(platelet derived growth factor)/VEGF growth factor family. The VEGFA gene is mapped to human chromosome 6p21.1. The encoded protein is a dimeric glycoprotein consisting two monomers antiparallel to each other. There are five types of VEGF ligands identified in mammals, which exists as different splice variants. Vascular endothelial growth factor 165 (VEGF165) belongs to the PDGF(platelet derived growth factor)/VEGF growth factor family and VEGF is secreted by many cell types.HumanKine VEGF165 is expressed in human HEK 293 cells as a glycosylated homodimer with an apparent molecular mass of 45 kDa. Production in human HEK 293 cells offers authentic glycosylation. Glycosylation contributes to stability in cell growth media and other applications.
Biochem/physiol Actions
VEGF (vascular endothelial growth factor) acts as a potent angiogenic factor and mitogen that stimulates proliferation, migration and formation of endothelial cells. VEGF stimulates permeabilization of blood vessels and is present in some tumors of the nervous system. VEGFA is known to interact with VEGFR co-receptors such as heparan sulphate proteoglycans and neuropilins. Mutation in the VEGFA gene results in defective vascular development. VEGF is induced by hypoxia, oncogene mutations, and cytokines such as (interleukin) IL-1, IL-8, TNF-α (tumor necrosis factor α).
Physical form
Lyophilized from a 0.2 μm filtered solution of 1X PBS
Analysis Note
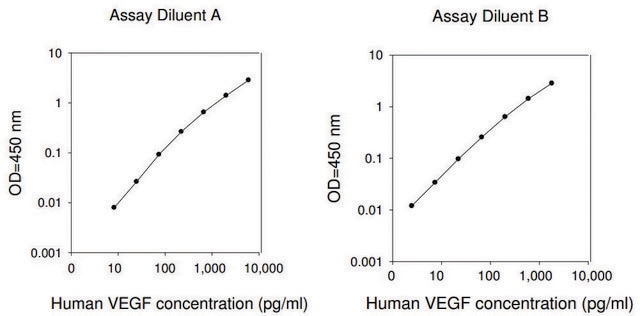
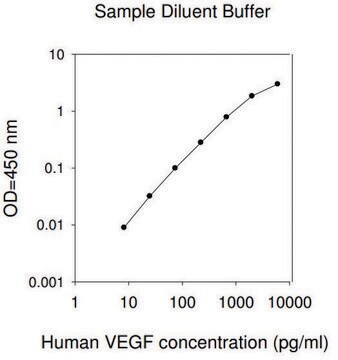
The specific activity was determined by the dose-dependent stimulation of the proliferation of HUVEC cells (human umbilical vein endothelial cells).
Legal Information
HumanKine is a registered trademark of Proteintech Group, Inc. and Humanzyme, Inc
comparable product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Genetic variants on chromosome 6p21.1 and 6p22.3
are associated with type 2 diabetes risk: a case?control
study in Han Chinese
are associated with type 2 diabetes risk: a case?control
study in Han Chinese
Lu F, et al.
Journal of Human Genetics, 57.5 (2012)
Chunming Li et al.
Molecular and cellular biochemistry, 346(1-2), 173-178 (2010-09-30)
Cell adhesion is an important process during morphogenesis, differentiation, and homeostasis in cell biology. The role of vascular endothelial growth factor (VEGF) in cell adhesion of keratinocytes is unclear. In our study, a human keratinocyte cell line, HaCaT cells, which
Transcriptome-wide Landscape of Pre-mRNA
Alternative Splicing Associated with Metastatic Colonization
Alternative Splicing Associated with Metastatic Colonization
Lu ZX, et al.
Molecular Cancer Research (2014)
Satsuki Mochizuki et al.
Biochemical and biophysical research communications, 402(4), 651-657 (2010-10-26)
ADAM28, a member of the ADAM (a disintegrin and metalloproteinase) gene family, is over-expressed by carcinoma cells and the expression correlates with carcinoma cell proliferation and progression in human lung and breast carcinomas. However, information about substrates of ADAM28 is
Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability
Fukumura D, et al.
Proceedings of the National Academy of Sciences of the USA, 98.5, 2604-2609 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service