I8250
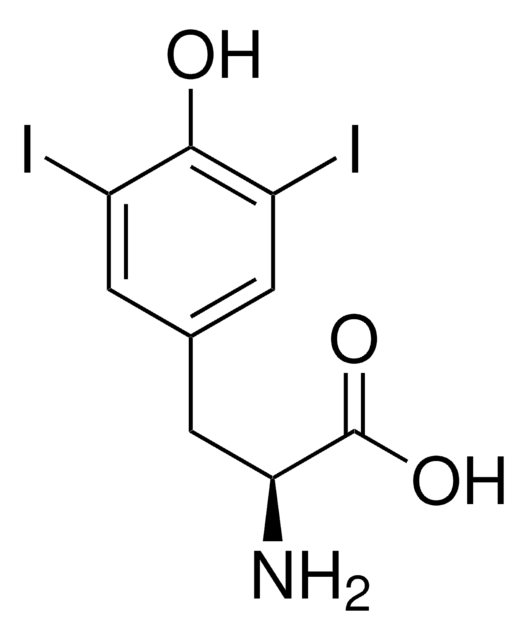
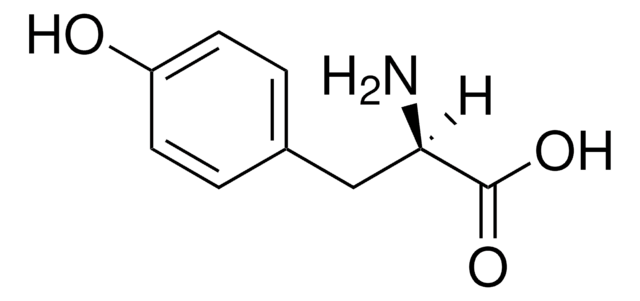
3-Iodo-L-tyrosine
Synonym(s):
3-Monoiodo-L-tyrosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H3-4-(OH)CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
307.09
Beilstein/REAXYS Number:
2941266
EC Number:
MDL number:
UNSPSC Code:
12352200
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
impurities
~5% tyrosine
mp
210 °C (dec.) (lit.)
solubility
dilute aqueous acid: soluble
storage temp.
−20°C
SMILES string
N[C@@H](Cc1ccc(O)c(I)c1)C(O)=O
InChI
1S/C9H10INO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)/t7-/m0/s1
InChI key
UQTZMGFTRHFAAM-ZETCQYMHSA-N
Gene Information
human ... TH(7054)
Looking for similar products? Visit Product Comparison Guide
General description
Iodotyrosine coupled with di-iodotyrosine results in the synthesis of 3,5,3′-tri-iodothyronine (T3) or 3,3′,5′-tri-iodothyronine (rT3).
Application
3-Iodo-L-tyrosine has been used as an inhibitor for tyrosine hydroxylase enzyme in Drosophila and silkworm pupae.
Biochem/physiol Actions
3-iodotyrosine (3-IY) inhibits tyrosine hydroxylase that catalyzes levodopa (L-DOPA) formation from tyrosine. Iodotyrosine deiodinase enzyme deficiency leads to elevated levels of 3-IY in serum and urine in severe hypothyroidism and goiter.
TH (tyrosine 3-hydroxylase) is responsible for catalyzing the first step of the noradrenergic biosynthesis pathway. Mutations in TH are associated with tyrosine hydroxylase deficiency, leading to conditions such as infantile parkinsonism and DOPA (dopamine)-responsive dystonia as well as encephalopathy with perinatal onset.
Tyrosine hydroxylase inhibitor.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jacqueline Studer et al.
Journal of forensic sciences, 59(6), 1650-1653 (2014-07-01)
Catecholamines, especially noradrenalin, are essential in the control of respiration and arousal. Thus, an impaired production of these neurotransmitters may contribute to the occurrence of sudden infant death syndrome (SIDS). The first step of the noradrenergic synthesis pathway is catalyzed
L G Harsing et al.
Neuroscience, 77(2), 419-429 (1997-03-01)
Striatal slices from the rat were preincubated with [3H]GABA and superfused in the presence of nipecotic acid and aminooxyacetic acid, inhibitors of high-affinity GABA transport and GABA aminotransferase, respectively. GABA efflux was estimated by monitoring tritium efflux, 98% of which
Mutations in the iodotyrosine deiodinase gene and hypothyroidism
Moreno J, et al.
The New England Journal of Medicine, 358(17), 1811-1818 (2008)
Anionic iodotyrosine residues are required for iodothyronine synthesis
De Vijlder JJ and den Hartog MT
European Journal of Endocrinology, 138(2), 227-231 (1998)
P F Fitzpatrick
Biochemistry, 30(15), 3658-3662 (1991-04-16)
The steady-state kinetic mechanism for rat tyrosine hydroxylase has been determined by using recombinant enzyme expressed in insect tissue culture cells. Variation of any two of the three substrates, tyrosine, 6-methyltetrahydropterin, and oxygen, together at nonsaturating concentrations of the third
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service