L7035
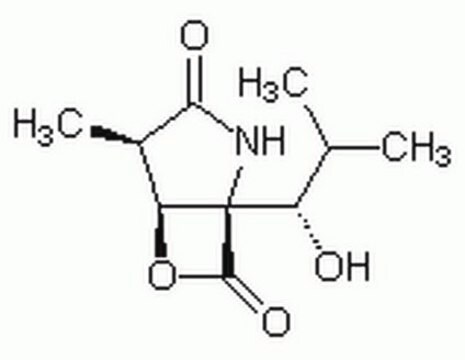
clasto-Lactacystin β-lactone
Synonym(s):
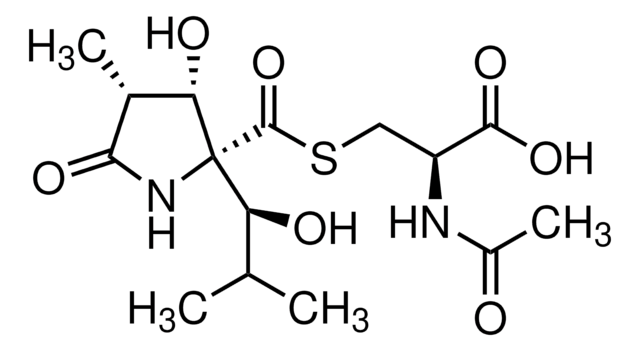
(1R,4R,5S)-1-[(1S)-1-Hydroxy-2-Methylpropyl]-4-Methyl-6-Oxa-2-Azabicyclo[3.2.0]Heptane-3,7-Dione, Omuralide
Select a Size
Select a Size
About This Item
Recommended Products
assay
≥95% (HPLC)
Quality Level
form
film
solubility
DMSO: soluble 25 mg/mL
storage temp.
−20°C
SMILES string
[H][C@@]12OC(=O)[C@@]1(NC(=O)[C@@H]2C)[C@@H](O)C(C)C
InChI
1S/C10H15NO4/c1-4(2)6(12)10-7(15-9(10)14)5(3)8(13)11-10/h4-7,12H,1-3H3,(H,11,13)/t5-,6+,7+,10-/m1/s1
InChI key
FWPWHHUJACGNMZ-NBBQQVJHSA-N
Application
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service