L9250
Leu-Ala hydrate
Bulk package
Synonym(s):
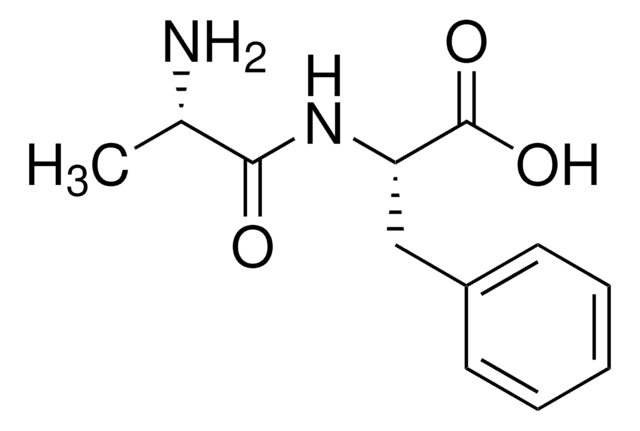
L-Leucyl-L-alanine hydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2CH(NH2)CONHCH(CH3)COOH · xH2O
CAS Number:
Molecular Weight:
202.25 (anhydrous basis)
Beilstein/REAXYS Number:
1726165
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
assay
≥98% (TLC)
form
powder
technique(s)
ligand binding assay: suitable
color
white
storage temp.
2-8°C
SMILES string
O.CC(C)C[C@H](N)C(=O)N[C@@H](C)C(O)=O
InChI
1S/C9H18N2O3.H2O/c1-5(2)4-7(10)8(12)11-6(3)9(13)14;/h5-7H,4,10H2,1-3H3,(H,11,12)(H,13,14);1H2/t6-,7-;/m0./s1
InChI key
FTDFAGANYJVHDA-LEUCUCNGSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Leucylalanine (Leu-Ala) is useful to produce non-electrolytic triorganotin (IV) derivatives (R3Sn(HL)) to study the models of metal-protein interactions.
Application
Leu-Ala hydrate has been used as a substrate in Tris-hydrochloride (HCl) buffer to analyze the leucine-alanine peptidase (LAP) activity in larvae.
Leucylalanine (Leu-Ala) is use to make non-electrolytic triorganotin(IV) derivatives (general formulae R3Sn(HL)) to study models of metal-protein interactions.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
David S Milner et al.
Proceedings of the National Academy of Sciences of the United States of America, 116(12), 5613-5622 (2019-03-08)
Many microbes acquire metabolites in a "feeding" process where complex polymers are broken down in the environment to their subunits. The subsequent uptake of soluble metabolites by a cell, sometimes called osmotrophy, is facilitated by transporter proteins. As such, the
New triorganotin (IV) derivatives of dipeptides as models for metal-protein interactions: synthesis, structural characterization and biological studies.
Nath M, Pokharia S, Eng G, Song X, Kumar A.
Spectrochimica Acta Part A: Molecular Spectroscopy, 63, 66-75 (2006)
Arun K Ghosh et al.
Journal of medicinal chemistry, 50(10), 2399-2407 (2007-04-17)
Structure-based design and synthesis of a number of potent and selective memapsin 2 inhibitors are described. These inhibitors were designed based upon the X-ray structure of memapsin 2-bound inhibitor 3 that incorporates methylsulfonyl alanine as the P2-ligand and a substituted
L P Biały et al.
Folia histochemica et cytobiologica, 40(2), 135-136 (2002-06-12)
We have used the dipeptide Leu-Ala in an attempt to prevent the formation of ubiquitin-protein conjugates in U937 cells by inhibition of cellular E3 enzymes (ubiquitin ligases). Proteasome inhibitors induce the formation of perinuclear aggregates of ubiquitinated proteins and proteasomes
Marie Terpager et al.
Journal of receptor and signal transduction research, 29(5), 235-245 (2009-09-15)
7TM receptors are easily fused to proteins such as G proteins and arrestin but because of the fact that their terminals are found on each side of the membrane they cannot be joined directly in covalent dimers. Here, we use
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service