N6877
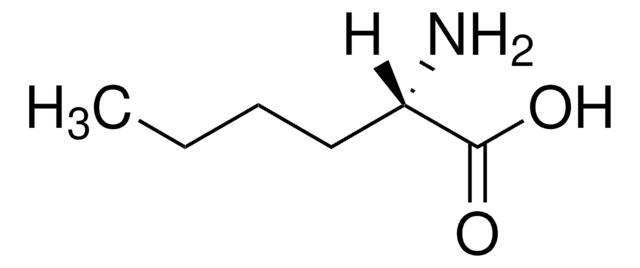
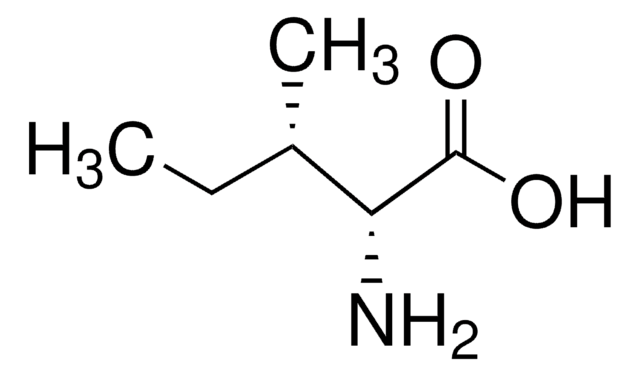
L-Norleucine
≥98% (TLC)
Synonym(s):
(S)-(+)-2-Aminohexanoic acid, (S)-2-Aminocaproic acid
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
assay
≥98% (TLC)
form
powder
color
white
mp
>300 °C (lit.)
application(s)
detection
SMILES string
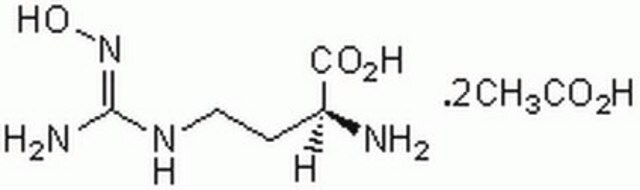
CCCC[C@H](N)C(O)=O
InChI
1S/C6H13NO2/c1-2-3-4-5(7)6(8)9/h5H,2-4,7H2,1H3,(H,8,9)/t5-/m0/s1
InChI key
LRQKBLKVPFOOQJ-YFKPBYRVSA-N
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | 114R-1 | 102R-1 | 123R-2 |
|---|---|---|---|
| conjugate unconjugated | conjugate unconjugated | conjugate unconjugated | conjugate unconjugated |
| species reactivity human | species reactivity human | species reactivity human | species reactivity human |
| biological source rabbit | biological source rabbit | biological source rabbit | biological source rabbit |
| clone EP3622, monoclonal | clone EPR3653, monoclonal | clone EP222, monoclonal | clone EP75, monoclonal |
| form buffered aqueous solution | form buffered aqueous solution | form buffered aqueous solution | form buffered aqueous solution |
application
- l-norleucine on high glucose-induced insulin sensitivity and mitochondrial function in skeletal muscle cells. This study explores the beneficial effects of L-Norleucine on insulin sensitivity and mitochondrial function, underlining its potential in metabolic pathway research and its application in managing diabetes through targeted amino acid therapy (Ding et al., 2024).
Biochem/physiol Actions
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service