H0377
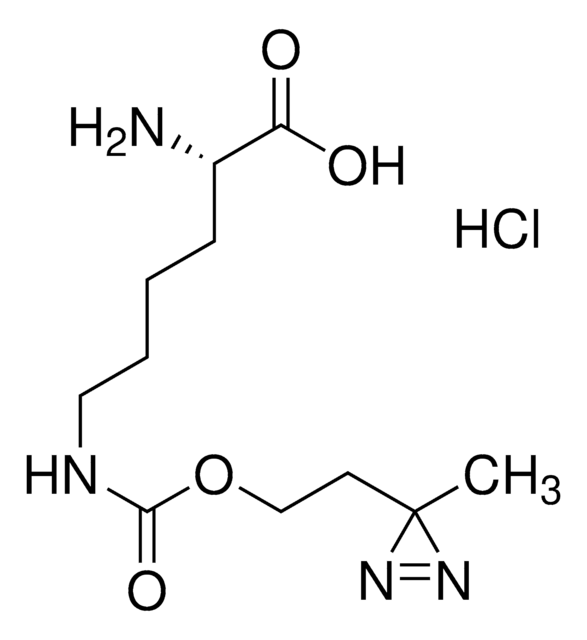
DL-5-Hydroxylysine hydrochloride
≥98% (TLC)
Synonym(s):
2,6-Diamino-5-hydroxycaproic acid hydrochloride, 2,6-Diamino-5-hydroxyhexanoic acid hydrochloride
About This Item
Recommended Products
Product Name
DL-5-Hydroxylysine hydrochloride,
assay
≥98% (TLC)
Quality Level
form
powder
color
white
mp
225 °C (dec.) (lit.)
application(s)
detection
peptide synthesis
SMILES string
Cl.NCC(O)CCC(N)C(O)=O
InChI
1S/C6H14N2O3.ClH/c7-3-4(9)1-2-5(8)6(10)11;/h4-5,9H,1-3,7-8H2,(H,10,11);1H
InChI key
MJXVOTKVFFAZQJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Characterization of acetyl-CoA: L-lysine N6-acetyltransferase, which catalyses the first step of carbon catabolism from lysine in Saccharomyces cerevisiae.: This research investigates the enzyme acetyl-CoA: L-lysine N6-acetyltransferase, which initiates the catabolism of lysine in Saccharomyces cerevisiae. Utilizing DL-5-Hydroxylysine hydrochloride, the study provides insights into the metabolic pathways and regulatory mechanisms of lysine degradation, contributing to the broader understanding of amino acid metabolism in yeast (Bode et al., 1993).
Biochem/physiol Actions
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service