SCP0225
Proteasome Substrate
≥95% (HPLC), lyophilized
Synonym(s):
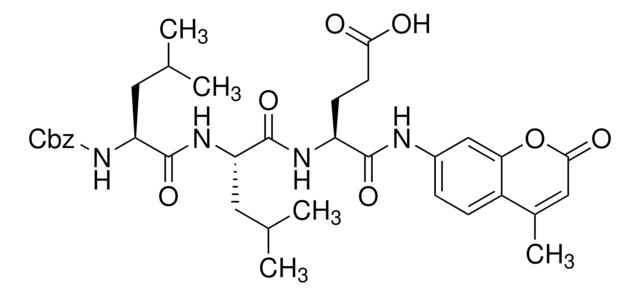
Carbobenzoxy-Gly-Gly-Leu-7-amido-4-methylcoumarin, benzyloxycarbonyl-glycyl-glycyl-leucyl-7-amido-4-methylcoumarin, Z-GGL-AMC
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C28H32N4O7
Molecular Weight:
536.58
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
product name
Proteasome Substrate,
assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥87%
storage condition
protect from light
storage temp.
−20°C
Amino Acid Sequence
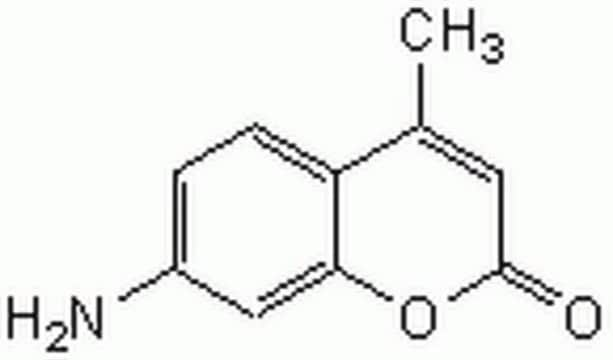
Z-Gly-Gly-Leu-AMC
Application
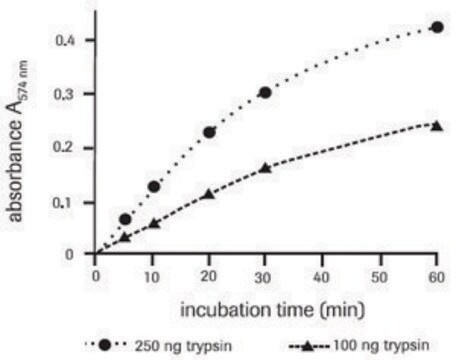
Z-Gly-Gly-Leu-7-amido-4-methylcoumarin (Z-Gly-Gly-Leu-AMC) has been used as a substrate for proteasome peptidase to measure proteosome activities using spectrophotometer.
Biochem/physiol Actions
Z-Gly-Gly-Leu-7-amido-4-methylcoumarin (Z-Gly-Gly-Leu-AMC) is a fluorogenic peptide that is used in analysis of protease and peptidase activity of proteasomes. Z-GGL-AMC has been noted as a particular substrate for chymotrypsin-like activity. It has low solubility and precipitates at 100μM.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Rohrwild et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(12), 5808-5813 (1996-06-11)
We have isolated a new type of ATP-dependent protease from Escherichia coli. It is the product of the heat-shock locus hslVU that encodes two proteins: HslV, a 19-kDa protein similar to proteasome beta subunits, and HslU, a 50-kDa protein related
C P Ma et al.
The Journal of biological chemistry, 267(15), 10515-10523 (1992-05-25)
A protein that greatly stimulates the multiple peptidase activities of the 20 S proteasome (also known as macropain, the multicatalytic protease complex, and 20 S protease) has been purified from bovine red blood cells and from bovine heart. The activator
S G Roudiak et al.
Biochemistry, 37(1), 377-386 (1998-02-07)
We have charterized a Mycobacterium smegmatis gene encoding a homolog of the ATP-dependent protease Lon (La). Our identification of a Lon homolog, in conjunction with our previous work, identifies M. smegmatis as the first known example of a eubacterium containing
István Nagy et al.
Journal of bacteriology, 185(2), 496-503 (2003-01-04)
In a proteasome-lacking mutant of Streptomyces coelicolor A3(2), an intracellular enzyme with chymotrypsin-like activity, absent from the wild type, was detected. Complementation that restored proteasome function did not suppress expression of the endopeptidase. Since the enzyme was not found in
Jung Wook Lee et al.
The Journal of biological chemistry, 284(48), 33475-33484 (2009-10-06)
HslVU is a bacterial ATP-dependent protease distantly related to eukaryotic proteasomes consisting of hexameric HslU ATPase and dodecameric HslV protease. As a homolog of the 20 S proteasome beta-subunits, HslV also uses the N-terminal threonine as the active site residue.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service