SML4058
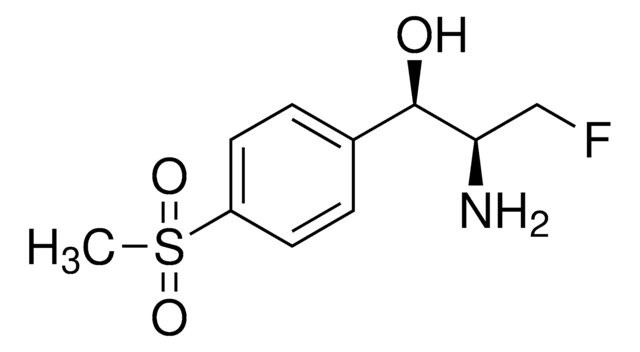
Florfenicol
≥98% (HPLC)
Synonym(s):
(-)-Florfenicol, 2,2-Dichloro-N-((1R,2S)-3-fluoro-1-hydroxy-1-(4-(methylsulfonyl)phenyl)propan-2-yl)acetamide, 2,2-Dichloro-N-[(1S,2R)-1-(fluoromethyl)-2-hydroxy-2-[4-(methylsulfonyl)phenyl]ethyl]acetamide, SCH 25298
About This Item
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
-10 to -25°C
SMILES string
ClC(C(N[C@@H]([C@H](O)C1=CC=C(S(C)(=O)=O)C=C1)CF)=O)Cl
InChI
1S/C12H14Cl2FNO4S/c1-21(19,20)8-4-2-7(3-5-8)10(17)9(6-15)16-12(18)11(13)14/h2-5,9-11,17H,6H2,1H3,(H,16,18)/t9-,10-/m1/s1
InChI key
AYIRNRDRBQJXIF-NXEZZACHSA-N
Biochem/physiol Actions
Florfenicol is a broad-spectrum antibiotic that is primarily used in veterinary medicine to treat respiratory infections in cattle and swine, and to prevent Salmonella infection in poultry. It has favorable ADME properties and is a fluorinated analog of chloramphenicol that has good tissue penetration and is resistant to chloramphenicol acetyltransferase inactivation. Florfenicol inhibits ribosomal activity and effectively inhibits bacterial protein synthesis, which makes it effective against various bacteria. However, oral administration of florfenicol may alter animal microbiota.
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 2 - STOT RE 1
target_organs
Liver,Brain,Testes,Spinal cord,Blood,gallbladder
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service