SRP3055
Heregulin beta -1 human
Animal-component free, recombinant, expressed in E. coli, ≥98% (SDS-PAGE), ≥98% (HPLC), suitable for cell culture
Synonym(s):
NRG1-b1, Neuregulin1
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352202
NACRES:
NA.32
Recommended Products
biological source
human
recombinant
expressed in E. coli
assay
≥98% (HPLC)
≥98% (SDS-PAGE)
form
lyophilized
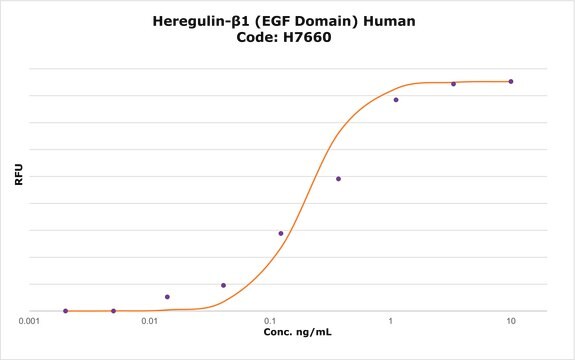
potency
≤0.5 ng/mL
mol wt
7.5 kDa
packaging
pkg of 50 μg
technique(s)
cell culture | mammalian: suitable
impurities
<0.1 EU/μg endotoxin, tested
color
white to off-white
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... NRG1(3084)
General description
Neuregulin/Heregulin is a family of structurally related polypeptide growth factors derived from alternatively spliced genes (NRG1, NRG2, NRG3 and NRG4). HRG1-β1 (heregulin-β1, also referred to as NRG1-β1) contains an Ig (immunoglobulin) domain and an EGF (epidermal growth factor)-like domain that is necessary for direct binding to receptor tyrosine kinases erb3 and erb4. The gene HRG1-β1 is mapped to human chromosome 8p12. Recombinant human Heregulin-β1 is a 7.5 kDa polypeptide consisting of only the EGF domain of heregulin-β1 (65 amino acid residues).
Application
Heregulin beta -1 human has been used to enhance proliferation of human dermal fibroblasts (hDFs).
Heregulin-β1 human has been used in the culture medium for the maintenance of Schwann cells. It has been used to study P-Rex1 (PI(3,4,5)P3-dependent Rac exchanger 1)-driven fibroblast invasiveness.
Biochem/physiol Actions
To date, there are over 14 soluble and transmembrane proteins derived from the NRG1 (neuregulin1) gene. Proteolytic processing of the extracellular domain of the transmembrane NRG1 isoforms release soluble growth factors. HRG1-β1 (heregulin-β1, also referred to as NRG1-β1) binding to receptor tyrosine kinases erb3 and erb4 induces erb3 and erb4 heterodimerization with erb2, stimulating intrinsic kinase activity, which leads to tyrosine phosphorylation. Although HRG1-β1 biological effects is still unclear, it has been found to promote motility and invasiveness of breast cancer cells which may also involve up-regulation of expression and function of the autocrine motility-promoting factor (AMF).
Sequence
SHLVKCAEKE KTFCVNGGEC FMVKDLSNPS RYLCKCPNEF TGDRCQNYVM ASFYKHLGIE FMEAE
Physical form
Lyophilized with no additives.
Reconstitution
Centrifuge the vial prior to opening. Reconstitute in water to a concentration of 0.1-1.0 mg/ml. Do not vortex. This solution can be stored at 2-8°C for up to 1 week. For extended storage, it is recommended to further dilute in a buffer containing a stabilizer (example 5% Trehalose) and store in working aliquots at -20°C to -80°C.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Heregulin-induced activation of ErbB3 by EGFR tyrosine kinase activity promotes tumor growth and metastasis in melanoma cells.
Ueno Y
International Journal of Cancer. Journal International Du Cancer, 123, 340-347 (2008)
The HER2- and heregulin ?1 (HRG)-inducible TNFR superfamily member Fn14 promotes HRG-driven breast cancer cell migration, invasion, and MMP9 expression.
Asrani K
Molecular Cancer Research, 11, 393-404 (2013)
Enhancement of wound healing efficiency mediated by artificial dermis functionalized with EGF or NRG1
Yoon D, et al.
Biomedical Materials (Bristol, England), 13(4), 045007-045007 (2018)
Control of peripheral nerve myelination by the beta-secretase BACE1.
Willem M
Science, 314, 664-666 (2006)
Neuregulins and cancer.
Montero JC
Clinical Cancer Research, 14, 3237-3241 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service