T7402
Paclitaxel
from Taxus brevifolia, ≥95% (HPLC), powder
About This Item
Recommended Products
biological source
Taxus brevifolia
assay
≥95% (HPLC)
form
powder
color
white
mp
213 °C (dec.) (lit.)
solubility
DMSO: 50 mg/mL (can be stored frozen for several months)
methanol: soluble 50 mg/mL, clear, colorless (undergoes transesterification)
ethanol: soluble
antibiotic activity spectrum
viruses
mode of action
DNA synthesis | interferes
originator
Bristol-Myers Squibb
storage temp.
2-8°C
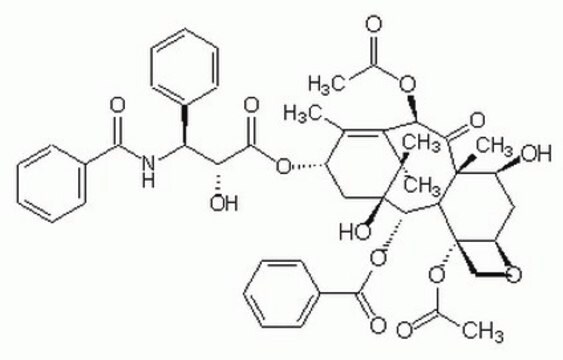
SMILES string
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)c6ccccc6)c7ccccc7
InChI
1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
InChI key
RCINICONZNJXQF-MZXODVADSA-N
Gene Information
human ... BCL2(596) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Features and Benefits
Packaging
Caution
Preparation Note
signalword
Danger
hcodes
Hazard Classifications
Muta. 2 - Repr. 1B - STOT RE 1 - STOT SE 2
target_organs
Central nervous system,Bone marrow,Cardio-vascular system, Eyes,Central nervous system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
High titer lentiviral particles including beta-actin, alpha-tubulin and vimentin used for live cell analysis of cytoskeleton structure proteins.
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service