1285910
USP
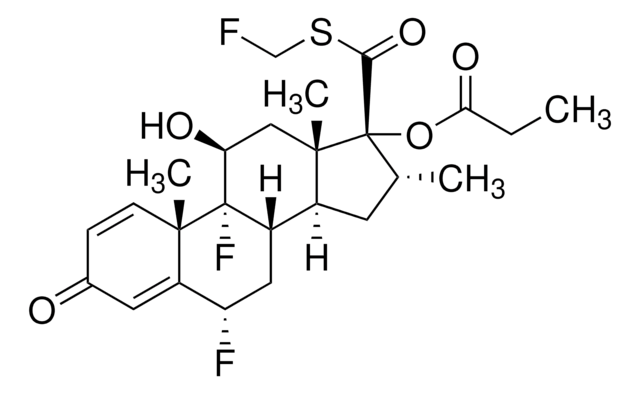
Fluticasone Propionate Nasal Spray Resolution Mixture
United States Pharmacopeia (USP) Reference Standard
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
fluticasone
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
mixture
storage temp.
2-8°C
General description
For further information and support please go to the website of the issuing Pharmacopoeia.
Application
- Fluticasone Propionate Cream
- Fluticasone Propionate Ointment
Analysis Note
Other Notes
Related product
signalword
Warning
hcodes
Hazard Classifications
Repr. 2 - STOT RE 2
target_organs
Endocrine system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Learn how the Purospher® STAR RP-18e Hibar® HPLC column meets the system suitability criteria outlined in the USP monograph for the fluticasone propionate assay.
Related Content
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service