UHPLC/MS Analysis of Antineoplastic and Antidepressant Drugs in Plasma on Titan C18 after SPE using HybridSPE®-PLus
Materials
SPE tube or plate
CONDITIONS
sample/matrix
rat plasma
SPE well plate
HybridSPE-PLus 96-Well Plate, 50 mg/2 mL per well (575659-U)
sample preparation
final analyte concentration 50 ng/mL
sample addition
400 μL of 1% formic acid acetonitile:spiked plasma (3:1) per well
column
Titan C18, 5 cm x 2.1 mm I.D., 1.9 μm particles (577122-U)
mobile phase
[A] 5 mM ammonium formate; [B] 5 mM ammonium formate in 90:10 (v:v) acetonitrile:water
gradient
35 to 90% B in 1.5 min, held at 90% B for 0.4 min
flow rate
0.4 mL/min
pressure
3582 psi (247 bar)
column temp.
35 °C
detector
TOF/MS
injection
1 μL
Description
Analysis Note
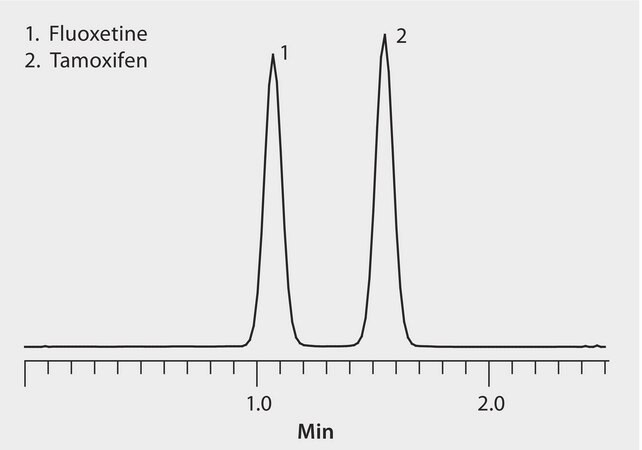
HybridSPE-Phospholipid plates removed plasma-derived proteins and phospholipids to maximize LC/MS sensitivity. Titan C18 U/HPLC columns provided rapid resolution. The highest grade LC-MS solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable identification and quantification.
Legal Information
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany
null