LC/MS/MS of 25-Dihydroxyvitamin D2, 25-Hydroxyvitamin D3, and 3-epi-25-Hydroxyvitamin D3 in Serum or Plasma on Ascentis® Express F5 after SPE with HybridSPE®-Phospholipid, Minimization of Matrix Effects
Materials
analytical column
SPE tube or plate
standard
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
plasma or serum spiked at 25 ng/mL with each vitamin D metabolite
SPE well plate
575656-U (HybridSPE-Phospholipid 96-well plate, 50 mg/2 mL)
sample addition
100 μL spiked rat plasma followed by 300 μL 1% formic acid in acetonitrile
elution
apply vacuum at 10 in Hg for 4 min
eluate post-treatment
collect filtrate and analyze directly
column
Ascentis Express F5, 10 cm x 2.1 mm I.D., 2.7 μm particles (53569-U)
mobile phase
(A) 5 mM ammonium formate in 75:25 (v/v) methanol:water
flow rate
0.4 mL/min
column temp.
40 °C
detector
MS, ESI(+), m/z 100-1000; phospholipids monitored at 496.3, 524.3, 758.5, 786.5, 806.5, and 810.5 m/z
injection
1 μL
Description
Analysis Note
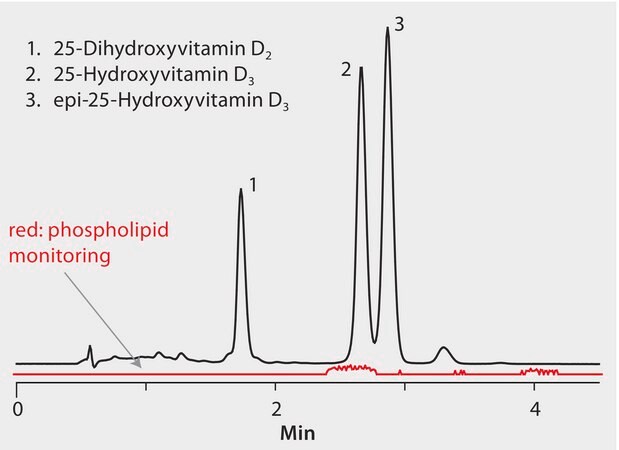
Chromatographic resolution of the various homologs of vitamin D2 is necessary for accurate quantification, especially considering several key metabolites are isobaric and not distinguishable by MS alone. A pentafluorophenyl HPLC phase (Ascentis Express F5) was chosen because of its ability to rapidly resolve the vitamin D homologs tested, especially the 25-hydroxyvitamin D3 and the 3-epi-25-hydroxyvitamin D3 that coelute on C18 stationary phases. HybridSPE-Phospholipid selectively depleted the phospholipid matrix and precipitated proteins, providing no interference from the serum matrix. Cerilliant CRMs provided reliable quantification.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany