PEPPSI™ Catalysts Overview
PEPPSI™-IPr: An air- and water-stable catalysts for industrially useful intermolecular cross-coupling reactions, aminations, and intramolecular Heck transformations.
Introduction
Professor Mike Organ at York University, along with co-workers Dr. Chris O’Brien and Dr. Eric Kantchev, have developed an elegant palladium N-heterocyclic-carbene (NHC) catalyst system built around a simple concept.1 They reacted PdCl2 with a bulky NHC ligand, 2,6-diisopropylphenyllimidazolium chloride (IPr), and an α-donating 3-chloropyridine ligand for stability. The title complex, PEPPSI™, stands for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation.
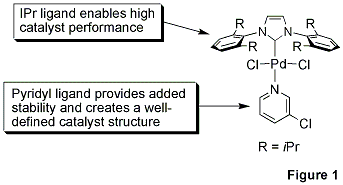
The 3-chloropyridyl ligand functions as a ‘throw-away’ ligand, while the bulky IPr ligand improves reductive elimination of the substrate which in turn increases TONs (Figure 1).2 The α-donating power of the NHC ligands also binds the metal more tightly than traditional phosphines and thus prevents metal dissociation. We are proud to offer gram-scale quantities of the PEPPSI™ catalyst in our collaboration with the Organ research group. The efficient mediation of C-C and C-N bond-forming processes, robust stability, and competitive cost structure make it attractive for widespread application in the research and fine chemical arena.
Advantages of the PEPPSI™-IPr Catalyst Family
- Extremely stable to air and moisture
- Commercialized on kilo scale
- Improved or comparable activity to known Pd catalysts
- High performance in various reaction paradigms
- Many reactions occur at room temperature
- No need for additional ligands ⇒ one component catalyst
- Cost-competitive
Stability and Handling
Unlike traditional palladium phosphine and NHC catalysts, PEPPSI™ is robust and can be stored indefinitely outside an inert atmosphere. The catalyst may be weighed out on bench utilizing normal methods and can even be subjected to a water workup without observable decomposition by 1H NMR. Perhaps most impressively, PEPPSI™ has been heated in dimethylsulfoxide at 120°C for hours without decomposition and subsequent deactivation of the catalyst. This Pd(II) complex becomes active in situ through reduction to the Pd(0)-NHC active catalyst – thus it can be considered a ligand stabilized Pd(PPh3)4 surrogate, minus the handling deficiencies.
PEPPSI™-IPr Representative Applications
Negishi Couplings
PEPPSI™-IPr (1) is a highly efficient and mild catalyst for forming alkyl-alkyl bonds, as illustrated in Figure 2. Sp3(RX)-sp3(RZnX) couplings mediated by 1 include a wide spectrum of functionality such as esters, nitriles, amides, and acetates (2–5).2
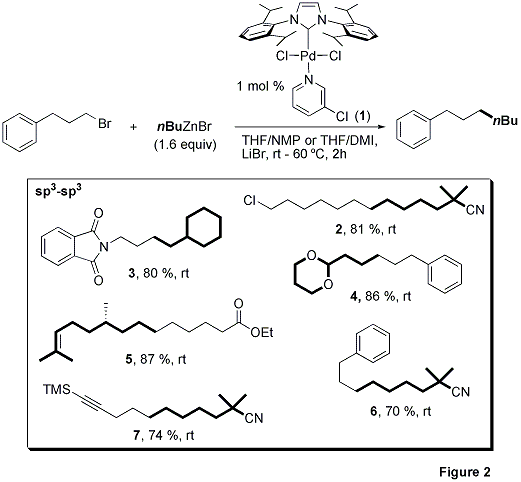
Notably the terminal alkynyl TMS group in compound 7 is completely stable to the cross-coupling of an alkyl chloride under room temperature reaction conditions. These results lend credence to the possibility of coupling substrates that contain biologically active components and subsequent expeditious synthesis of natural product intermediates. The wide range of alkyl bromides, chlorides, and tosylates supported by the PEPPSI™ system extend the general usefulness (compounds 2 – 7) of this reaction paradigm. Incredibly, the Organ research group successfully achieved the coupling of a bromide in the presence of a chloride by judicious choice of reaction conditions (compound 2). Note that we have added an anhydrous solution of LiCl in THF (667544) in order to facilitate rapid deployment of this additive for PEPPSI™ mediated Negishi Couplings.
Suzuki Couplings
PEPPSI™-IPr can be used effectively with a wide range of electron-rich (deactivated) and electron-poor (activated) substrates.3 The high activity of this catalyst system in the Suzuki coupling presents a strong case for application in industrial and academic research laboratories on a global scale.
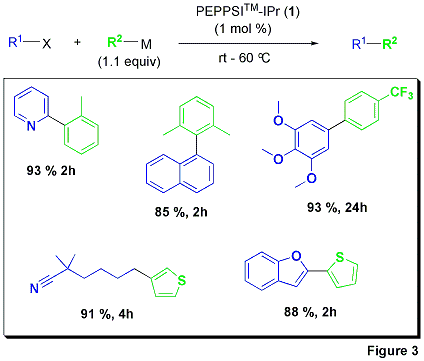
All Suzuki reactions were accomplished using typical laboratory preparations without the need for glove-box handling. The PEPPSI™-IPr pre-catalyst was weighed out in the air and activated in situ under a blanket of inert gas. The Organ group performed a full evaluation of heteroatom and electronically varied reaction partners. The reactions of various boronic acids proceeded smoothly in reagent-grade isopropanol and potassium t-butoxide was found to be the optimal base to ensure high product conversions. Thus, the broad utility of PEPPSI™-IPr was demonstrated in the production of a complex array of organic building blocks in high isolated yields (Figure 3).
Buchwald-Hartwig Aminations
The Organ group was pleased to discover that PEPPSI™-IPr is an excellent catalyst for the palladium-catalyzed cross-coupling of aryl chlorides and bromides with amines.4
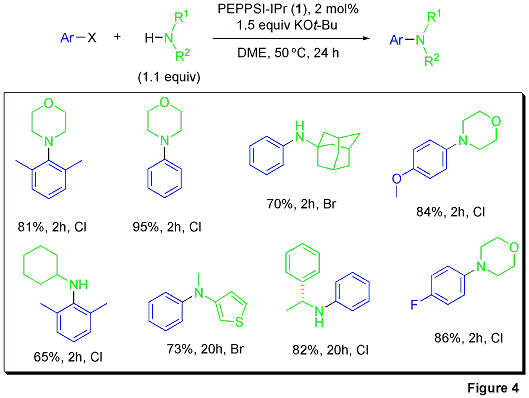
The results in Figure 4 indicate that use of this catalyst system allows for the successful arylation of various amines with superb yields. Morpholine, arylamines, and even adamantylamine undergo facile amination to afford a variety of aryl- and biarylamines. It is worth noting that the mild reaction conditions (temperature and base) tolerate electron-rich, electron-poor, and heteroaromatic substrates. This finding also shows that Pd-NHC complexes are not only viable as catalysts, but in many cases manifest tremendous efficiency and atom-economy in aromatic C—N bond-forming processes.
Kumada Couplings
Many studies have been performed on the oxidative addition of aryl halides with Pd(0) and subsequent coupling of Grignard reagents, however deficiencies in these previous examples include high catalyst loadings, high temperatures, and the necessity of aryliodide substrates to reach adequate conversions.5 The Organ group has reported Kumada couplings of various aryl chlorides with Grignard reagents (Figure 5).6 These room-temperature oxidative additions of aryl chlorides equal the best results to date. Reactions with 1-2 mol % PEPPSI™-IPr in THF/DME (1:1) at room-temperature produced the respective biaryl organics in excellent yields.
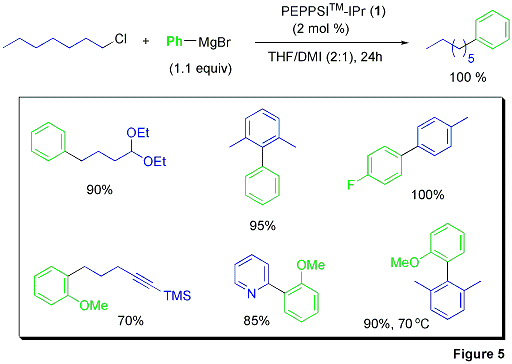
Also note that both electron-rich and electron-poor Grignard reagents underwent reaction as well as sterically hindered aryl chlorides. This mild Kumada protocol shows superior tolerance of ether, TMS, and alkynyl functionalities. Functionalized 5-aryl-substituted indoles are produced in good yields.
Tandem Methodology
A mild, new protocol expands the scope of the PEPPSI™-IPr catalyst in C—N bond-forming processes even further. As a complement to the well-known Fischer indole synthesis,7 wherein an N-acyl hydrazone is transformed into the indole architecture through a sigmatropic rearrangement, the Organ group has reacted a vinyl halide with various 2-bromoanilines in the presence of PEPPSI™-IPr to afford 2-substituted indoles in good yields (Figure 6).8
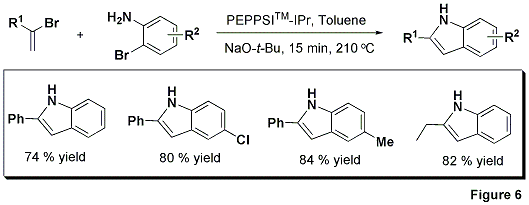
The PEPPSI™-IPr catalyst highlights our drive to advance science for our clients through expansion of our proprietary catalyst portfolio, including the Grubbs’ Nobel prize-winning metathesis technology. PEPPSI™-IPr is distinguished by higher efficiency and superior functional group tolerance, and meets or exceeds the performance of traditional phosphine systems in industrially useful Suzuki, Negishi, and Buchwald-Hartwig reactions.
PEPPSI™-SIPr is a novel state-of-the-art catalyst that successfully performs cross-coupling niche reactions. Note that this Pd-NHC catalyst only differs from PEPPSI™-IPr in that the NHC ligand backbone is saturated, thus providing additional flexibility and substrate control under specific reaction conditions. The Organ group has invented a readily employed Kumada protocol utilizing the PEPPSI™ catalyst system, which enables the rapid production of a diverse array of drug-like heterocycles. Challenging substrate combinations are readily converted into industrially useful biaryl and amine building blocks suitable for employment in the synthesis of more complex architectures (Figure 7).

For instance, the high reactivity of PEPPSI™-SIPr has promoted the synthesis of a tetra-ortho-substituted heterocycle for the first time utilizing any known catalytic methodology (Figure 8). This spectacular result, when combined with the observed reaction rates,9 indicate that PEPPSI™-SIPr slightly outperforms PEPPSI™-IPr in the Kumada reaction.

This PEPPSI™ catalyst addition offers unprecedented scope, reactivity, and stability advantages in the Kumada and Buchwald-Hartwig reactions. Note that in the former case, a wide range of heteroatom-containing reaction partners were coupled in excellent yield. Notably, the synthesis of the naphthylol derivative shown in Figure 9 was successfully achieved on a 10 g scale in 85% yield, further cementing the industrial viability of this system. The Organ research group has also committed to demonstrating the power of this system in coupling large molecular weight fragments, which might be rationally applied to late-stage natural product syntheses.
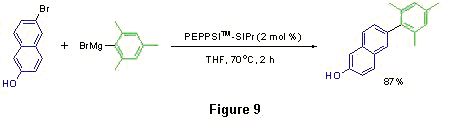
Conclusion
The PEPPSI™ catalyst family highlights our drive to advance science for our clients through expansion of our proprietary catalyst portfolio, including the Grubbs’ Nobel prize-winning metathesis technology. PEPPSI™-IPr is distinguished by higher efficiency and superior functional group tolerance, and meets or exceeds the performance of traditional phosphine systems in industrially useful Suzuki, Negishi, and Buchwald-Hartwig reactions.
References
To continue reading please sign in or create an account.
Don't Have An Account?