HPLC Separation of Nitrosamines with SupelTM Carbon LC
Eddy Tan, Associate Senior Scientist
R&D APAC Lab Singapore
Abstract
The SupelTM Carbon LC column is used for the separation of six nitrosamine compounds. This method is a reversed phase gradient separation of the nitrosamines for identification and quantification.
Introduction
Nitrosamines have been known to exist in the environment and within food sources1, 2 with awareness further raised in 2018 when many regulatory authorities for medicines became aware of the dangers of N-Nitrosamines as drug contaminants.3 This class of compounds is known to be potent carcinogens4 and can be introduced through multiple introduction pathways.5 In this application, 2.7 µm porous graphitic carbon particles in SupelTM Carbon LC column are used to separate six different nitrosamines followed by UV detection.
Experimental
For this study, the following procedures and conditions were used:
Mobile Phase Preparation
- Water + 0.1% trifluoroacetic acid (TFA): Add 100 µL of TFA to 100 mL of water.
- Acetonitrile + 0.1% TFA: Add 100 µL of TFA to 100 mL of acetonitrile, add trifluoroacetic acid
Nitrosamine Standard Solutions for Linearity, LOD and LOQ Determination
NMBA, NEIPA, NDIPA, NDBA and NMPA
- For NMBA, NEIPA, NDIPA, NDBA and NMPA (standard concentration ~1000 mg/L), transfer 400 µL into a 5 mL volumetric flask.
- Top-up to mark with mobile phase A (water + 0.1% TFA) and mix well. Sonicate for 10 min. This solution is the main working standard (~80 mg/L).
- Dilute to approximately 5, 10, 20, 40, 60 mg/L with mobile phase A in 5 mL volumetric flasks based on serial dilution. (True concentrations for linearity are based on batch CoA of reference materials used.)
NDEA
- For NDEA (Neat liquid ~99.8 % purity), weigh ~50 mg of NDEA CRM into a 5 mL volumetric flask.
- Top-up to mark with acetonitrile and mix well. Sonicate for 10 min. This solution is the stock solution (~10,000 mg/L).
- Dilute to approximately 5, 10, 20, 40, 60, 80 mg/L with mobile phase A in 5 mL volumetric flasks based on serial dilution. (True concentrations for linearity is based on batch CoA of reference material used.)
Linearity data is obtained from linear regression, LOD and LOQ values are calculated based on USP.
Results & Discussion
The analysis of the standard mix containing six nitrosamine impurities (NDEA, NMBA, NEIPA, NDIPA, NDBA, NMPA) at 20 mg/mL each is shown in Figure 1. The chromatographic data for the analysis of the standard mix is listed in Table 2. All peaks are base line separated.
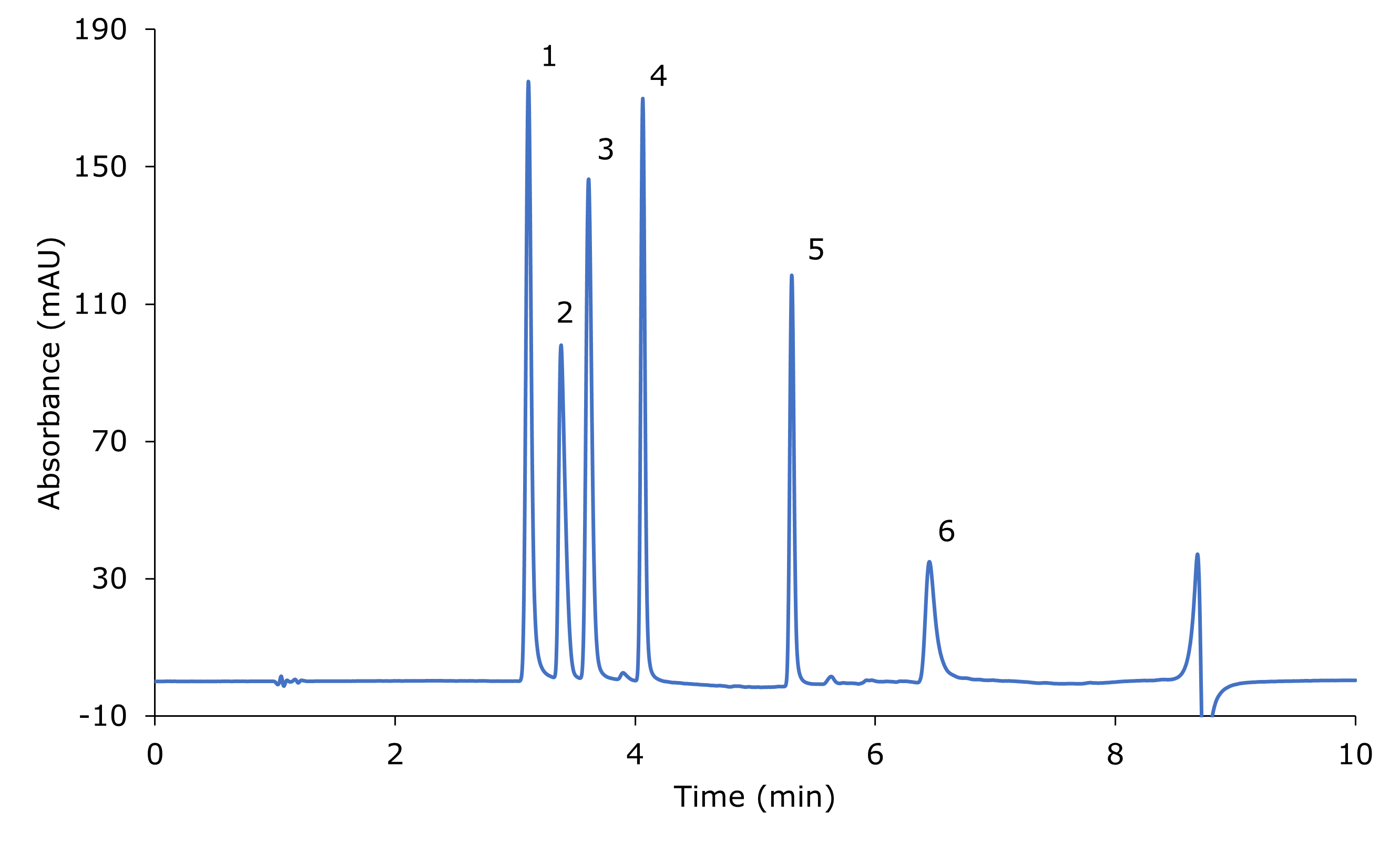
Figure 1.Separation of six nitrosamines standard mix (~20 mg/L each); blank subtracted chromatogram. 1: NDEA, 2: NMBA, 3: NEIPA, 4: NDIPA, 5: NDBA, 6: NMPA.
Calibration
The calibration data summary and combined limits of detection (LOD) and limits of quantification (LOQ) of the applied method for the six nitrosamines (NDEA, NMBA, NEIPA, NDIPA, NDBA, NMPA) are listed in Table 3. All six nitrosamines exhibited excellent linear regression of more than 0.99. LOD and LOQ of these nitrosamines are found to be in low mg/L range when using UV detection.
Conclusion
The SupelTM Carbon LC column is able to separate six nitrosamines based on reversed phase with good reproducibility for repeated analyses (data not shown here). The resolution and peak asymmetry for the six different nitrosamines are greater than 1.5 and less than 2.0 respectively. LOD and LOQ of the tested nitrosamines are in the low mg/L range and can be further improved with the use of mass spectrometry instrumentation.
References
To continue reading please sign in or create an account.
Don't Have An Account?