Thrombin Factor IIa
Specificity
Thrombin is an endolytic serine protease that selectively cleaves the Arg--Gly bonds of fibrinogen to form fibrin and release fibrinopeptides A and B.1,2
The optimal cleavage sites for thrombin have been determined to be 1) A-B-Pro-Arg-||-X-Y where A and B are hydrophobic amino acids and X and Y are nonacidic amino acids and 2) Gly-Arg-||-Gly.2
Thrombin from any mammalian species will clot the fibrinogen of any other mammalian species.3
Thrombin cleaves fibrinogen in 2 ways, but only at arginine sites. The primary cleavage product, fibrinopeptide A is cleaved from fibrinogen after amino acid 16 and sometimes after amino acid 19, while a secondary cleavage product, fibrinopeptide B is produced by cleavage at amino acid 14.4
Thrombin is active in the pH range of 5-10.5
Catalytic optimum is pH 8.3.5
Thrombin precipitates at pH 5 or less.5
Thrombin does not require divalent metal ions or cofactors for activity. However, Na+-dependent allosteric activation of thrombin has been shown to play a role in defining the primary specificity of thrombin to cleave after Arg residues.6 Thromobmodulin serves as a cofactor to thrombin during the activation of protein C.7
In vivo Processing and Physical Properties
The predominant form of thrombin in vivo is it's zymogen, prothrombin (factor II), which is produced in the liver. The concentration of prothrombin in normal human plasma is ~5-10 mg/dL.8 Prothrombin is a glycoprotein with a glycan content of ~12%.8
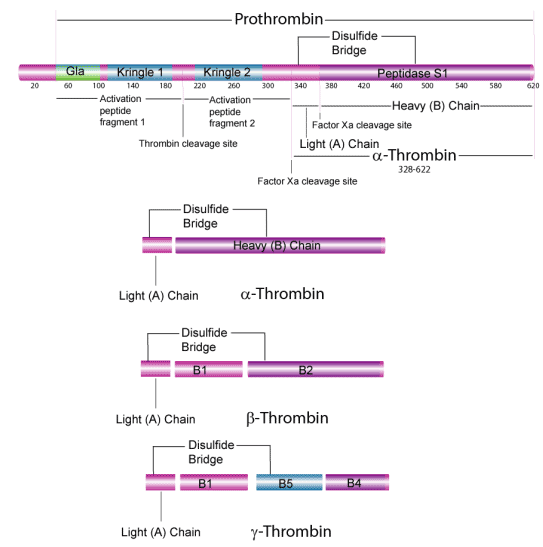
Prothrombin is cleaved in vivo by activated factor X releasing the activation peptide and cleaving thrombin into light and heavy chains yielding catalytically active α-thrombin. α-Thrombin is composed of a light chain (A chain)(MW ~ 6,000) and a heavy chain (B chain)(~31,000). These two chains are joined by one disulfide bond.12 The B chain of human thrombin consists of a peptide portion (MW 29,485) and a carbohydrate portion (2334) with N-linked glycosylation at three Asn sites.9,10 Bovine thrombin contains 1.7% glucosamine, 1.8% sialic acid, 0.61% galactose, and 0.95% mannose.11 Thrombin also contains γ-carboxyglutamyl residues. These modified glutamyl residues are the result of carboxylation by a microsomal enzyme, vitamin K-dependent carboxylase. γ-carboxyglutamyl residues are necessary for the calcium-dependent interaction with a negatively charged phospholipid surface, which is essential for the conversion of prothrombin to thrombin.12 In vivo, prothrombin is activated on the surface of a phospholipid membrane that binds the amino terminus of prothrombin along with factors Va and Xa. The activation process starts slowly because factor V itself has to be activated by the initial small amounts of thrombin.
Under certain storage conditions, autolytic digestion of α-thrombin results in the formation of β- and γ-thrombins that lack fibrinolytic activity, but retain some activity against synthetic peptide substrates and protein substrates other than fibrinogen.13 Our thrombin preparations are predominantly the α-thrombin form.
Human thrombin consists of several isozymes with isoelectric points in the range of 6.35-7.6.
For bovine the pI range is 7.05 - 7.114
E1%(280nm) = 18.3 (human)15
E1%(280nm) = 19.5 (bovine)16
Measurement of Thrombin Activity
Our thrombin assay procedure is expressed in NIH units obtained by direct comparison to a NIH Thrombin Reference Standard.
The NIH assay procedure uses 0.2 mL of diluted plasma (1:1 with saline) as a substrate and 0.1 mL of thrombin sample (stabilized in a 1% buffered albumin solution at pH 7.35) based on a modification of the method of Biggs.17 Only clotting times in the range of 15-25 seconds are used for determining thrombin concentrations.
Thrombin concentrations in the literature are typically reported in terms of different units of activity.18-20
Several conventions are used in thrombin literature:
1 WHO unit = 1 NIH unit
1 NIH unit = 1 USP unit
1 NIH unit = 0.324 +/- 0.073 µg
1 IOWA unit = 0.83 NIH unit
Thrombin (human and bovine) will catalyze the hydrolysis of several peptide p-nitroanilides, tosyl-arg-nitrobenzyl ester, and a thiobenzyl ester synthetic substrates.21
Applications
Production of fibrin clot in plasma:
Typicallty one to two units of thrombin will clot one mL of plasma.
Cleavage of Fusion Proteins:
Thrombin can be used for the cleavage of many peptides at the thrombin recognition site using concentrations of 0.5 NIH units thrombin per one nanomole polypeptide in 20 microliters of 50 mM ammonium bicarbonate, pH 8.0.22
Thrombin cleavage of fusion proteins can be carried out at a thrombin to fusion protein ratio of 1:500.23
Fusion proteins may be cleaved in thrombin cleavage buffer consisting of 50 mM Tris, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2 and 0.1% 2-mercaptoethanol. 2 mg of fusion protein was incubated with 4 µg of thrombin for 20 minutes at RT in the cleavage buffer.24
Substrates
Natural Substrates
Fibrinogen, Factor V,25 Factor VIII,25 Factor XIII,25 Thrombospondin,26 Prothrombin,12 and Protein C.27
Synthetic Substrates
N-Benzoyl-Phe-Val-Arg 4-methoxy-b-naphthylamide, N-Benzoyl-Phe-Val-Arg-p-nitroanilide, Boc-β-benzyl-Asp-Pro-Arg-7-amido-4-methylcoumarin, Boc-Val-Pro-Arg-7-amido-4-methylcoumarin, Sar-Pro-Arg p-nitroanilide, Thrombin generation chromogenic, N-p-Tosyl-Gly-Pro-Arg 7-amido-4-methylcoumarin, N-(p-Tosyl)-Gly-Pro-Arg p-nitroanilide.
Inhibitors
Diisopropylfluorophosphate,28 phenylmethylsulfonylfluoride, 28 AEBSF, hirudin,28 tetranitromethane,28 proflavine,28 antithrombin III,28 a1-antitrypsin28 , a1-antiplasmin,28 gabexate mesylate,29 antipain,30 and tosyl-L-lysinechloromethylketone.31
References
To continue reading please sign in or create an account.
Don't Have An Account?