すべての画像(1)
About This Item
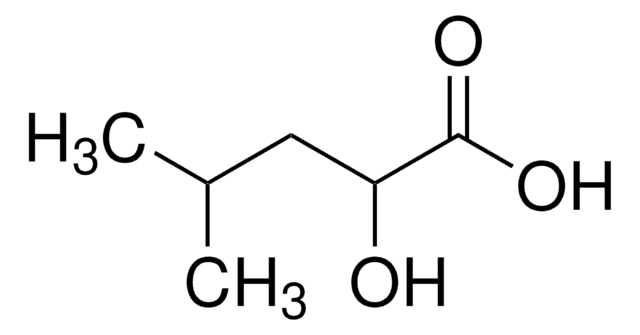
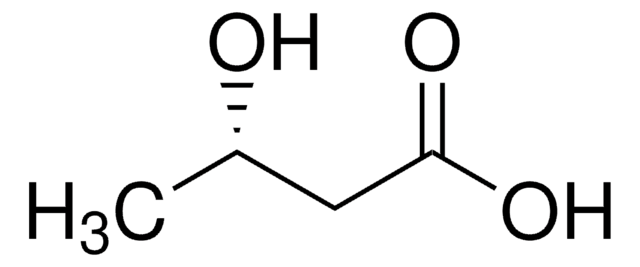
化学式:
(CH3)2CHCH2CH(OH)CO2H
CAS番号:
分子量:
132.16
EC Number:
MDL番号:
UNSPSCコード:
51113400
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
219827-VAR:
219827-BULK:
219827-1G:
この製品を見ている人はこちらもチェック
Antti A Mero et al.
Journal of the International Society of Sports Nutrition, 7, 1-1 (2010-01-07)
Alfa-Hydroxy-isocaproic acid (HICA) is an end product of leucine metabolism in human tissues such as muscle and connective tissue. According to the clinical and experimental studies, HICA can be considered as an anti-catabolic substance. The present study investigated the effects
Takayoshi Awakawa et al.
Nature communications, 9(1), 3534-3534 (2018-09-01)
Reprogramming of the NRPS/PKS assembly line is an attractive method for the production of new bioactive molecules. However, it is usually hampered by the loss of intimate domain/module interactions required for the precise control of chain transfer and elongation reactions.
Fernanda U Fontella et al.
Metabolic brain disease, 17(1), 47-54 (2002-03-16)
In this study we investigated the in vitro effects of the metabolites accumulating in maple syrup urine disease on lipid peroxidation in brain of young rats. Chemiluminescence and thiobarbituric acid-reactive substances were measured in brain homogenates from 7- and 30-day-old
H Drechsel et al.
Journal of bacteriology, 175(9), 2727-2733 (1993-05-01)
Growth promotion and iron transport studies revealed that certain alpha-keto acids generated by amino acid deaminases, by enterobacteria of the Proteus-Providencia-Morganella group (of the tribe Proteeae), show significant siderophore activity. Their iron-binding properties were confirmed by the chrome azurol S
K D Webster et al.
Archives of biochemistry and biophysics, 273(2), 562-571 (1989-09-01)
L-Thiomorpholine-3-carboxylic acid (L-TMC) is a cyclized analog of S-(2-chloroethyl)-L-cysteine, which is cytotoxic in vitro and nephrotoxic in vivo. To determine whether L-TMC may play a role in S-(2-chloroethyl)-L-cysteine-induced toxicity, the cytotoxicity of L-TMC was studied in isolated rat kidney cells.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)