すべての画像(1)
About This Item
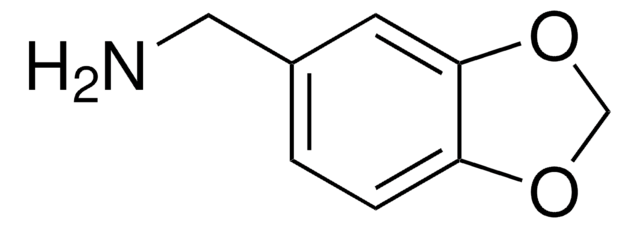
実験式(ヒル表記法):
C9H11NO2 · HCl
CAS番号:
分子量:
201.65
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
アッセイ:
98%
おすすめの製品
品質水準
アッセイ
98%
mp
216-218 °C (lit.)
官能基
amine
SMILES記法
Cl.NCCc1ccc2OCOc2c1
InChI
1S/C9H11NO2.ClH/c10-4-3-7-1-2-8-9(5-7)12-6-11-8;/h1-2,5H,3-4,6,10H2;1H
InChI Key
NDYXFQODWGEGNU-UHFFFAOYSA-N
詳細
3,4-Methylenedioxyphenethylamine hydrochloride can be synthesized by reacting aluminum chloride, LiAlH4 and 3,4-methylenedioxyphenylacetonitrile.
アプリケーション
3,4-Methylenedioxyphenethylamine hydrochloride may be used to synthesize N-(3,4-methylenedioxyphenethyl)-2-(3-bromo-4-methoxyphenyl)acetamide.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
560529-BULK:
560529-5G:
560529-1G:
560529-VAR:
この製品を見ている人はこちらもチェック
An aryne route to laureline, and related topics.
Gibson MS, et al.
J. Chem. Soc. Sect. C, 16, 2234-2238 (1970)
Jan G Bruhn et al.
Journal of psychoactive drugs, 40(2), 219-222 (2008-08-30)
Human interest in psychoactive phenethylamines is known from the use of mescaline-containing cacti and designer drugs such as Ecstasy. From the alkaloid composition of cacti we hypothesized that substances resembling Ecstasy might occur naturally. In this article we show that
D J De Silva et al.
Neuroscience, 134(4), 1363-1375 (2005-08-02)
Substituted amphetamines such as p-chloroamphetamine and the abused drug methylenedioxymethamphetamine cause selective destruction of serotonin axons in rats, by unknown mechanisms. Since some serotonin neurones also express neuronal nitric oxide synthase, which has been implicated in neurotoxicity, the present study
Milica Ninković et al.
Nephrology (Carlton, Vic.), 13(1), 33-37 (2008-01-18)
The mechanism of MDMA (3,4-methylenedioxymethamphetamine)-induced toxicity is believed to be, in part, due to enhanced oxidative stress. As MDMA is eliminated via the kidney, the aim of this study was to investigate whether MDMA created conditions of oxidative stress within
J F Bagli et al.
Journal of medicinal chemistry, 19(7), 876-882 (1976-07-01)
Synthesis of a series of thienylethanolamines having varying substituents on the thiophene ring and on the nitrogen atom is described using the general procedure reported earlier. In the determination of their pharmacological profile, some of the derivatives showed marked antihypertensive
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)