おすすめの製品
アッセイ
≥95%
フォーム
powder
利用可能性
available only in USA
保管温度
2-8°C
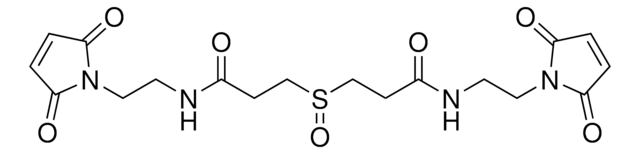
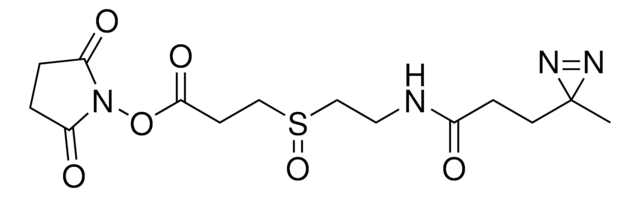
SMILES記法
[S](=O)(CCC(=O)ON2C(=O)CCC2=O)CCC(=O)ON1C(=O)CCC1=O
InChI Key
XJSVVHDQSGMHAJ-UHFFFAOYSA-N
アプリケーション
DSSO (disuccinimidyl sulfoxide) crosslinker is a homobifunctional, amine-targeting, sulfoxide-containing crosslinker for analysis of protein-protein interactions (PPIs) through crosslinking mass spectrometry (XL-MS). Membrane-permeable DSSO possesses two N-hydroxysuccinimide (NHS) esters for targeting Lys, a 10.1 Å spacer arm, and two symmetrical C-S cleavable bonds adjacent to the central sulfoxide. The post-cleavage spacer yields tagged peptides for unambiguous identification by collision-induced dissociation in tandem MS. DSSO Crosslinker provides complementary data to thiol-reactive and acidic residue-targeting reagents and will find wide utility in the elucidation of PPIs, study of proteins that function as complexes, quantification of structural dynamics, and the quest for targeting ″undruggable″ protein targets.
その他情報
Technology Spotlight: Cross-Linkers for Elucidation of Protein-Protein Interactions
Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes
Structural dynamics of the human COP9 signalosome revealed by cross-linking mass spectrometry and integrative modeling
Developing a Multiplexed Quantitative Cross-Linking Mass Spectrometry Platform for Comparative Structural Analysis of Protein Complexes
Development of a Novel Sulfoxide-Containing MS-Cleavable Homobifunctional Cysteine-Reactive Cross-Linker for Studying Protein–Protein Interactions
Developing an Acidic Residue Reactive and Sulfoxide-Containing MS-Cleavable Homobifunctional Cross-Linker for Probing Protein-Protein Interactions
Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes
Structural dynamics of the human COP9 signalosome revealed by cross-linking mass spectrometry and integrative modeling
Developing a Multiplexed Quantitative Cross-Linking Mass Spectrometry Platform for Comparative Structural Analysis of Protein Complexes
Development of a Novel Sulfoxide-Containing MS-Cleavable Homobifunctional Cysteine-Reactive Cross-Linker for Studying Protein–Protein Interactions
Developing an Acidic Residue Reactive and Sulfoxide-Containing MS-Cleavable Homobifunctional Cross-Linker for Probing Protein-Protein Interactions
法的情報
Subject to US Patent #9,222,943 and US Patent Application #15/275,001 of the Regents of the University of California
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Self-react. C
保管分類コード
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
909602-BULK:
909602-100MG:
909602-VAR:
最新バージョンのいずれかを選択してください:
Tara K Bartolec et al.
Analytical chemistry, 92(2), 1874-1882 (2019-12-19)
Saccharomyces cerevisiae has the most comprehensively characterized protein-protein interaction network, or interactome, of any eukaryote. This has predominantly been generated through multiple, systematic studies of protein-protein interactions by two-hybrid techniques and of affinity-purified protein complexes. A pressing question is to
Clinton Yu et al.
Analytical chemistry, 88(20), 10301-10308 (2016-10-19)
Cross-linking mass spectrometry (XL-MS) represents a recently popularized hybrid methodology for defining protein-protein interactions (PPIs) and analyzing structures of large protein assemblies. In particular, XL-MS strategies have been demonstrated to be effective in elucidating molecular details of PPIs at the
Christian E Stieger et al.
Journal of proteome research, 18(3), 1363-1370 (2019-01-30)
Cross-linking mass spectrometry is becoming increasingly popular, and current advances are widening the applicability of the technique so that it can be utilized by nonspecialist laboratories. Specifically, the use of novel mass-spectrometry-cleavable (MS-cleavable) reagents dramatically reduces the complexity of the
Athit Kao et al.
Molecular & cellular proteomics : MCP, 10(1), M110-M110 (2010-08-26)
Knowledge of elaborate structures of protein complexes is fundamental for understanding their functions and regulations. Although cross-linking coupled with mass spectrometry (MS) has been presented as a feasible strategy for structural elucidation of large multisubunit protein complexes, this method has
Sebastiaan C de Graaf et al.
Journal of proteome research, 18(2), 642-651 (2018-12-24)
Protein interactions enable much more complex behavior than the sum of the individual protein parts would suggest and represents a level of biological complexity requiring full understanding when unravelling cellular processes. Cross-linking mass spectrometry has emerged as an attractive approach
資料
Sulfoxide-containing MS-cleavable cross-linkers capture protein-protein interactions in native cellular environments, aiding PPI identification.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)