935409
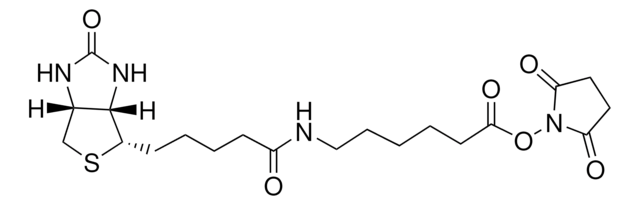
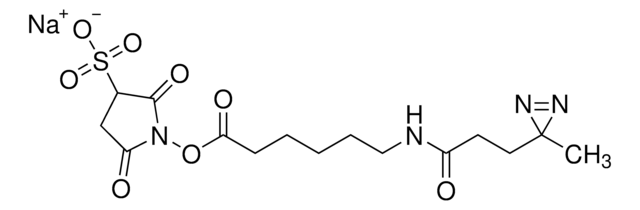
Sulfo-NHS-LC-LC-Biotin
≥95%
別名:
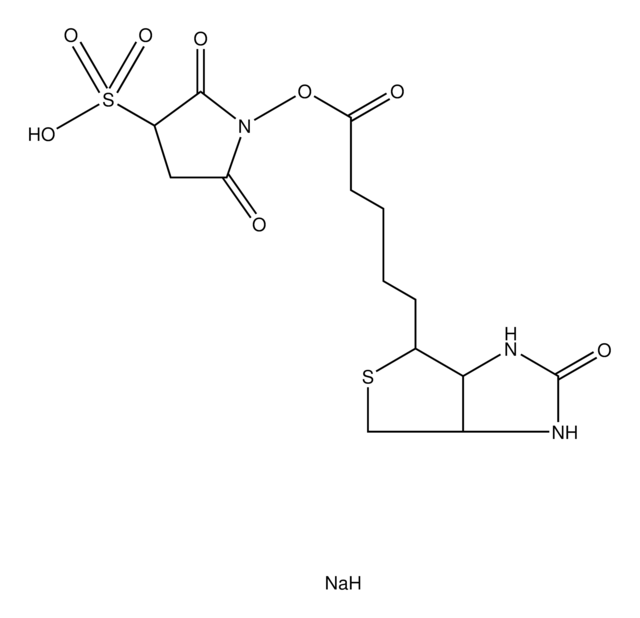
3-Pyrrolidinesulfonic acid, 1-[[6-[[6-[[5-(hexahydro-2-oxo-1H-thieno[3,4-d]imidazol-4-yl)-1-oxopentyl]amino]-1-oxohexyl]amino]-1-oxohexyl]oxy]-2,5-dioxo-, monosodium salt, [3aS-(3aα,4β,6aα)]-[partial]-, EZ-Link Sulfo-NHS-LC-LC-Biotin, Hexanoic acid, 6-[[6-[[5-[(3aS,4S,6aR)-hexahydro-2-oxo-1H-thieno[3,4-d]imidazol-4-yl]-1-oxopentyl]amino]-1-oxohexyl]amino]-, 2,5-dioxo-3-sulfo-1-pyrrolidinyl ester, sodium salt (1:1), Sulfo-NHS-XX-Biotin
About This Item
おすすめの製品
品質水準
アッセイ
≥95%
形状
solid
保管温度
−20°C
SMILES記法
[Na].O=C1NC2CSC(CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)ON3C(=O)CC(C3=O)S(=O)(=O)O)C2N1
InChI
InChI=1S/C26H41N5O10S2.Na/c32-20(27-14-8-2-4-12-23(35)41-31-22(34)15-19(25(31)36)43(38,39)40)10-3-1-7-13-28-21(33)11-6-5-9-18-24-17(16-42-18)29-26(37)30-24;/h17-19,24H,1-16H2,(H,27,32)(H,28,33)(H2,29,30,37)(H,38,39,40);/t17-,18-,19?,24-;/m0./s1
InChI Key
RWNPTJCDHAXLGI-BJPAGVOZSA-N
アプリケーション
特徴および利点
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
935409-50MG:
935409-25MG:
935409-BULK:
935409-VAR:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)