おすすめの製品
フォーム
methanol solution
包装
pkg of 1 × 1 mL (LM4113-1EA)
メーカー/製品名
Avanti Research™ - A Croda Brand
濃度
~10 μg/mL (Refer to C of A for lot specific concentration. )
アプリケーション
lipidomics
metabolomics
輸送温度
dry ice
保管温度
−20°C
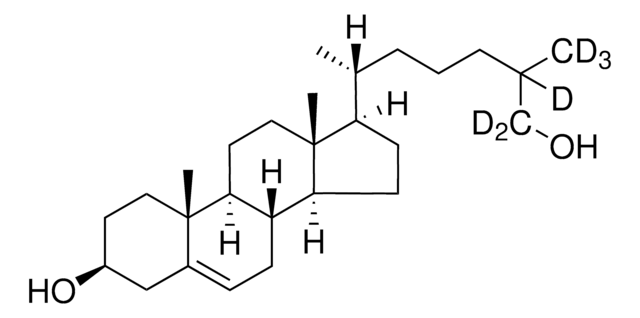
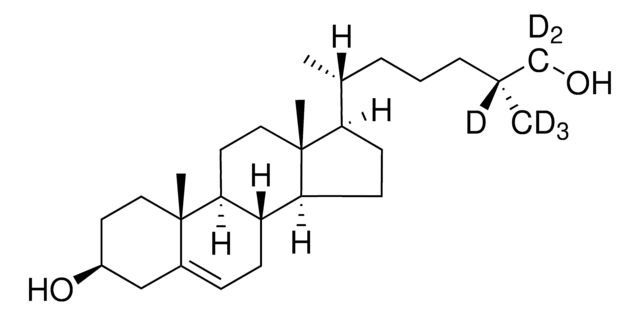
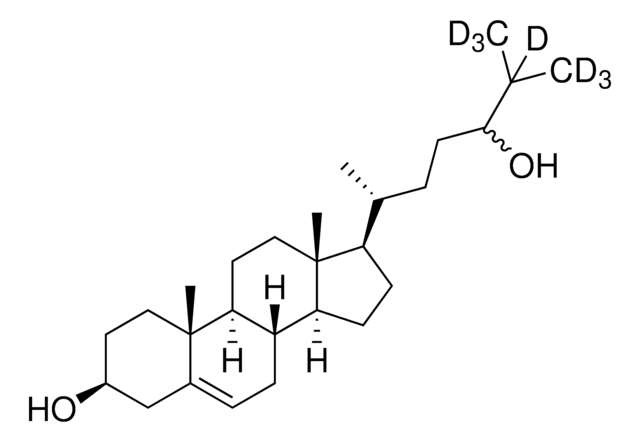
SMILES記法
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)C3=CC2)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCCC(O)(C([2H])([2H])[2H])C([2H])([2H])[2H]
InChI
1S/C27H46O2/c1-18(7-6-14-25(2,3)29)22-10-11-23-21-9-8-19-17-20(28)12-15-26(19,4)24(21)13-16-27(22,23)5/h8,18,20-24,28-29H,6-7,9-17H2,1-5H3/t18-,20+,21+,22-,23+,24+,26+,27-/m1/s1/i2D3,3D3
詳細
25-hydroxycholesterol is an oxygenated sterol and a hydroxylated derivative of cholesterol.
生物化学的/生理学的作用
25-hydroxycholesterol inhibits cholesterol biosynthesis by downregulating the function of 3-hydroxy-3-methylglutaryl-CoA reductase. It is found to be implicated in atherosclerosis. 25-hydroxycholesterol levels are known to be increased in hypercholesterolemic condition.
包装
2 mL Amber Glass Sealed Ampule (LM4113-1EA)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
ターゲットの組織
Eyes
保管分類コード
3 - Flammable liquids
WGK
WGK 2
引火点(°F)
49.5 °F - closed cup
引火点(℃)
9.7 °C - closed cup
最新バージョンのいずれかを選択してください:
Christopher M Adams et al.
The Journal of biological chemistry, 279(50), 52772-52780 (2004-09-29)
The current paper demonstrates that cholesterol and its hydroxylated derivative, 25-hydroxycholesterol (25-HC), inhibit cholesterol synthesis by two different mechanisms, both involving the proteins that control sterol regulatory element-binding proteins (SREBPs), membrane-bound transcription factors that activate genes encoding enzymes of lipid
Cholsoon Jang et al.
Nature medicine, 22(4), 421-426 (2016-03-08)
Epidemiological and experimental data implicate branched-chain amino acids (BCAAs) in the development of insulin resistance, but the mechanisms that underlie this link remain unclear. Insulin resistance in skeletal muscle stems from the excess accumulation of lipid species, a process that
Rosamaria Lappano et al.
PloS one, 6(1), e16631-e16631 (2011-02-10)
The hydroxylated derivatives of cholesterol, such as the oxysterols, play important roles in lipid metabolism. In particular, 25-hydroxycholesterol (25 HC) has been implicated in a variety of metabolic events including cholesterol homeostasis and atherosclerosis. 25 HC is detectable in human
Ken Cheng et al.
Journal of agricultural and food chemistry, 66(33), 8876-8884 (2018-07-26)
Membrane lipids, including sphingolipids and glycerol-phospholipids, are essential in maintaining the skin's barrier function in mammals, but their composition in fish skin and their response to diets have not been evaluated. This study investigated the impacts of reducing dietary eicosapentaenoic
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)