おすすめの製品
アッセイ
≥97% (HPLC)
品質水準
形状
powder
有効性
400 pM IC50
300 pM Ki
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
desiccated (hygroscopic)
protect from light
色
white
溶解性
DMSO: 50 mg/mL
water: insoluble
保管温度
−20°C
InChI
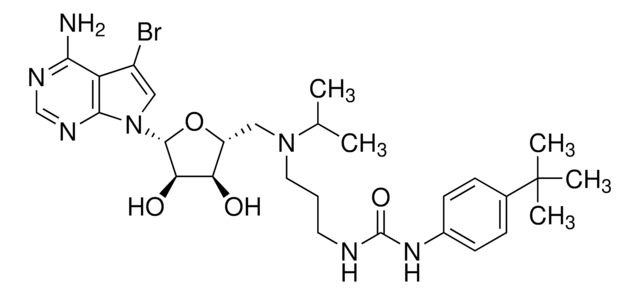
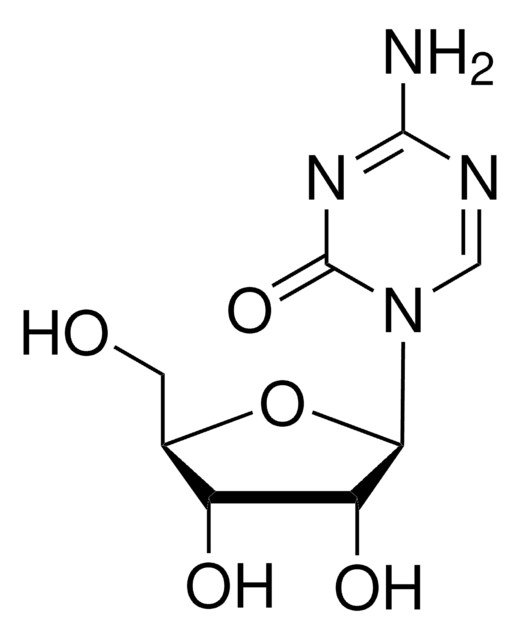
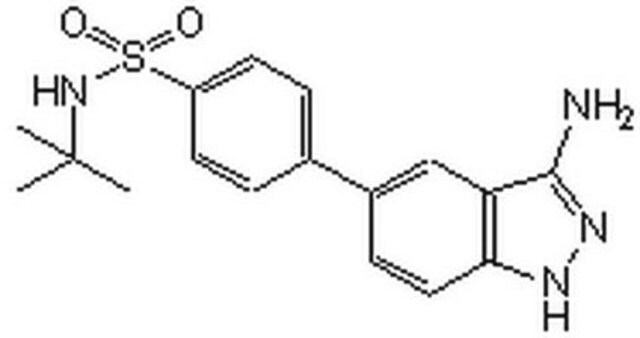
1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1
InChI Key
WXRGFPHDRFQODR-ICLZECGLSA-N
詳細
A cell-permeable S-adenosylmethionine (AdoMet/SAM) structural derivative that potently inhibits DOT1L methyltransferase activity (IC50 = 400 pM) in a SAM-competitive (Ki = 300 pM) manner via a 1:1 stoichiometric interaction (KD = 250 pM), not only blocking DOT1L SAM-binding site with its deazaadenosine moiety, but also disrupting the overall structural integrity of the catalytic pocket with its tert-butylphenyl moiety, while inhibiting PRMT5 only at much higher concentrations (IC50 = 521 nM or 25 µM, respectively, using free or MEP50-complexed PRMT5) and exhibiting little potency against CARM1, EHMT2, EZH1, EZH2, PRMT1, PRMT8, SETD7, or WHSC1 even at a high concentration of 50 µM. Although EPZ004777 effectively inhibits cellular H3K79 methylation regardless of MLL translocation status (ICmax ~3 µM post 4 d treatment in MV4-11, MOLM-13, and Jurkat cells with MLL-AF4, MLL-AF9, and no MLL rearrangement, respectively), the viability of MLL rearranged cells are more susceptible to EPZ004777 treatment (IC50 = 0.62 to 6.74 µM in cultures with MLL-AF4, MLL-AF9, or MLL-ENL fusion; GI50 =13.9 µM to no inhibition at 50 µM in cultures with no MLL rearrangement; by Guava ViaCount Assay/4000-0040 at the end of 14 to 18 treatment period; cells split with fresh media/inhibitor every 3-4 d) due to effective transcription blockage of MLL fusion target genes (IC50 ~700 nM in reducing HOXA9 & MEIS1 mRNA levels in MV411 & MOLM-13 cultures). Despite poor pharmacokinetic properties, continuous EPZ004777 infusion via subcutaneously implanted osmotic pumps (100 mg/mL & 150 mg/mL to achieve steady state plasma drug conc of 0.64 & 0.84 µM, respectively) is reported to significantly extend the survival of NSG mice received MV4-11 cells via tail vein injection (100% death on d11 or d17 post 14 d vehicle or drug infusion period, respectively).
A cell-permeable, non-toxic, a near chemical derivative of S-adenosylmethionine (SAM) that competitively binds to the SAM-binding pocket of DOT1L and inhibits its activity in a reversible manner (IC50 = 400 pM; Ki = 300 pM). Shown to inhibit the methylation of cellular H3K79 in MLL cells and block the expression of leukemogenic genes. Displays excellent selectivity over other histone methyltransferases PRMT5 (IC50 = 521 nM), CARM1, EHMT2, EZH1, EZH2, PRMT1, PRMT8, SETD7 and WHSC1 (IC50 >50 µM). Inhibits the proliferation of MV4-1, MOLM-13, KOPN-8 cells (IC50 = 170, 720, and 620 nM, respectively) and suppresses the growth of MV4-11 xenografts in nude mice model (50 mg/ml, s.c. minipump infusion). Blocks the cell cycle at G0/G1 phase and induces apoptotic cell death by activating caspase 3/7. Also shown to accelerate reprogramming of somatic cells into induced pluripotent stem cells.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
生物化学的/生理学的作用
Cell permeable: yes
Primary Target
DOT1L
DOT1L
Reversible: yes
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
調製ノート
Use only fresh DMSO for reconstitution.
再構成
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
その他情報
Daigle, S.R., et al. 2013. Blood.122, 1017.
Yu, W., et al. 2012. Nat. Commun.3, 1288.
Daigle, S.R., et al. 2011. Cancer Cell.20, 53.
Yu, W., et al. 2012. Nat. Commun.3, 1288.
Daigle, S.R., et al. 2011. Cancer Cell.20, 53.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)