MABS1253
Anti-O-GlcNAc Antibody, clone RL1
clone RL1, from mouse
別名:
β-GlcNAc, β-linked N-acetylglucosamine, O-GlcNAc, O-linked β-GlcNAc
About This Item
ICC
IF
IHC
IP
WB
affinity binding assay
inhibition assay
electron microscopy: suitable
immunocytochemistry: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
inhibition assay: suitable
western blot: suitable
おすすめの製品
由来生物
mouse
品質水準
抗体製品の状態
purified immunoglobulin
抗体製品タイプ
primary antibodies
クローン
RL1, monoclonal
化学種の反応性
human, Drosophila, Xenopus, rat
テクニック
affinity binding assay: suitable
electron microscopy: suitable
immunocytochemistry: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
inhibition assay: suitable
western blot: suitable
アイソタイプ
IgMκ
輸送温度
ambient
ターゲットの翻訳後修飾
glycosylation
遺伝子情報
human ... OGT(8473)
詳細
特異性
免疫原
アプリケーション
Affinity Binding Assay: A representative lot of clone RL1 & clone RL2 (Cat. No. MABS157) partially competed against each other for binding immobilized 180 kDa O-GlcNAcylated nuclear envelope protein (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
Electron Microscopy Analysis: A representative lot immunolocalized target proteins at the cytoplasmic and/or nucleoplasmic margins of the pore complex with no specific staining of the perinuclear space of isolated rat liver nuclear envelopes (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
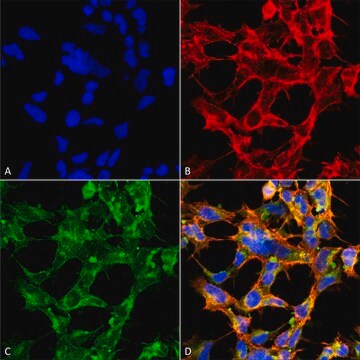
Immunocytochemistry Analysis: Representative lots stained the nuclear envelope of paraformaldehyde-fixed, Triton X-100-permeabilized HeLa cells, NRK normal rat kidney epithelial cells and isolated rat liver nuclei by fluorescent immunocytochemistry (Yang, L., et al. (1997). J. Cell Biol. 139(5):1077-1087; Byrd, D.A., et al. (1994). J. Cell Biol. 127(6 Pt 1):1515-1526; Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
Immunofluorescence Analysis: A representative lot stained the nuclear envelope proteins in formaldehyde-fixed, Triton X-100-permeabilzied salivary glands dissected from third-instar Drosophila larvae by fluorescent immunohistochemistry (Goldberg, M., et al. (1998). Mol. Cell. Biol. 18(7):4315-4323).
Immunofluorescence Analysis: Representative lots stained the nuclear envelope of methanol-fixed xenopus ovary and rat liver cryosections by fluorescent immunohistochemistry (Featherstone, C., et al. (1988). J. Cell Biol. 107(4):1289-1297; Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560).
Immunoprecipitation Analysis: A representative lot immunoprecipitated several nuclear envelope proteins in salt-washed nuclear envelope preparations from rat liver and NRK normal rat kidney epithelial cells. Galactosylation of GlcNAc on nuclear envelope proteins by galactosyltransferase treatment significantly inhibited the immunoadsorption of these nuclear pore complex glycoproteins by clone RL1 (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Inhibition Analysis: A representative lot, when injected in the vegetal hemisphere of xenopus oocyte cytoplasm, inhibited nucleoplasmin nuclear import in a dose-dependent manner without affecting myoglobin nuclear import or RNA export (Featherstone, C., et al. (1988). J. Cell Biol. 107(4):1289-1297).
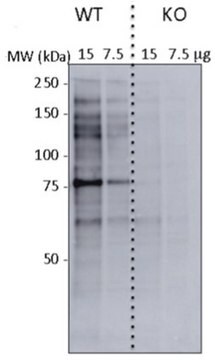
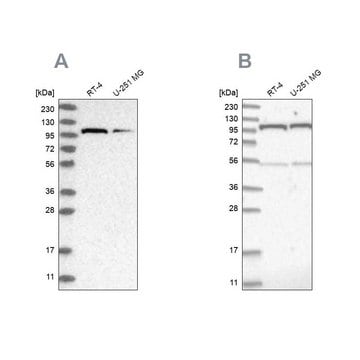
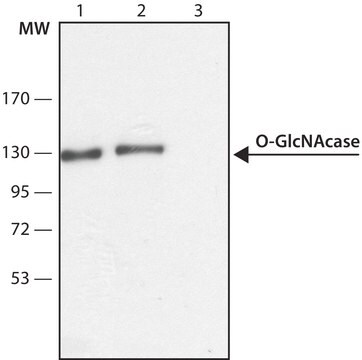
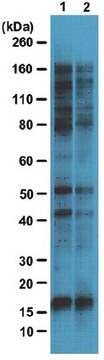
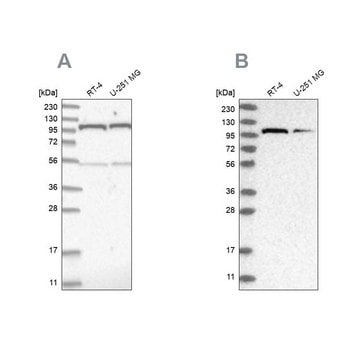
Western Blotting Analysis: Representative lots detected several nuclear envelope proteins in Xenopus oocyte nucleus extract and in salt-washed rat liver nuclear envelope preparations, including target bands of 210, 180, 145, 100, 63, 58, 54, and 45 kDa. GlcNAc removal by beta-N-acetylglucosaminidase treatment greatly reduced the detection of these nuclear pore complex glycoproteins by clone RL1 (Byrd, D.A., et al. (1994). J. Cell Biol. 127(6 Pt 1):1515-1526; Featherstone, C., et al. (1988). J. Cell Biol. 107(4):1289-1297; Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-11560; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
品質
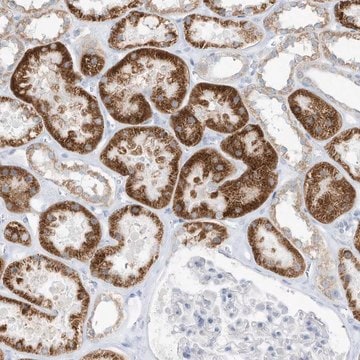
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected O-GlcNAcylated cytoplasmic and nuclear pore proteins in rat colon tissue sections.
ターゲットの説明
物理的形状
保管および安定性
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze
その他情報
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
保管分類コード
12 - Non Combustible Liquids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
MABS1253:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)