74011
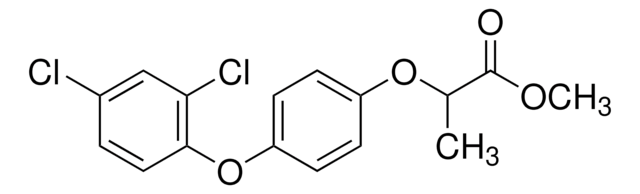
(±)-Diclofop
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
別名:
(RS)-2-[4-(2,4-Dichlorophenoxy)phenoxy]propionic acid
About This Item
おすすめの製品
グレード
certified reference material
TraceCERT®
品質水準
製品種目
TraceCERT®
シェルフライフ
limited shelf life, expiry date on the label
メーカー/製品名
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
フォーマット
neat
保管温度
2-8°C
SMILES記法
CC(Oc1ccc(Oc2ccc(Cl)cc2Cl)cc1)C(O)=O
InChI
1S/C15H12Cl2O4/c1-9(15(18)19)20-11-3-5-12(6-4-11)21-14-7-2-10(16)8-13(14)17/h2-9H,1H3,(H,18,19)
InChI Key
OOLBCHYXZDXLDS-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
(+/–)-Diclofop is a chiral herbicide that belongs to the chemical class of chiral aryloxyphenoxypropionate compounds. The actual herbicidal active ingredient― carboxylic acid, is released after application through hydrolysis of the ester. It is absorbed mainly through leaves and inhibits the biosynthesis of fatty acids by suppressing the activity of acetyl CoA carboxylase (ACCase). It is used for the post-emergence control of wild oats, wild millets, and other annual grass weeds commonly occurring in wheat, barley, rye, red fescue, and broad-leaved crops.
It was included on 1st June 2011 in Annex I of Directive 91/414/EEC by the European Commission Directive 2011/45/EU. It is authorized for use under EC Regulation No 1107/2009, as per the Commission Implementing Regulation (EU) No 540/2011, however it is a candidate for substitution.
アプリケーション
The (+/–)-Diclofop CRM can also be used as following:
- To evaluate the likely enantioselective oxidative stress produced in Microcystis aeruginosa by diclofop acid
- For analyzing the phytotoxic effects of diclofop acid enantiomers on the plant Arabidopsis thaliana
- To study the enantioselective toxicity of diclofop acid on the non-target rice Xiushui 63 seedlings
- In the chiral separation of diclofop-acid using one- and two- dimensional HPLC methods
- To determine the enantioselective toxicity and degradation of diclofop in three algal cultures
おすすめ製品
法的情報
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
74011-50MG:
74011-BULK:
74011-VAR:
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)