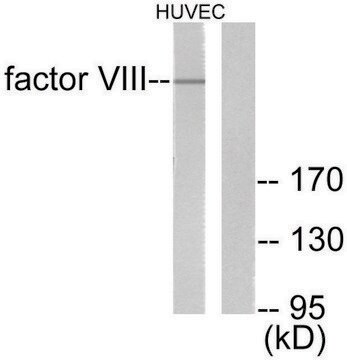
おすすめの製品
グレード
pharmaceutical primary standard
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Human coagulation factor VIII concentrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
免責事項
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
H0920000-1EA:
H0920000-5EA:
H0920000-3EA:
H0920000-37MG:
最新バージョンのいずれかを選択してください:
Elizabeth Duncan et al.
Methods in molecular biology (Clifton, N.J.), 992, 321-333 (2013-04-03)
Hemophilia A is an inherited bleeding disorder caused by a deficiency of factor VIII coagulant activity (FVIII:C). Patients are treated with infusions of either plasma-derived or recombinant factor VIII. However, some patients develop inhibitory antibodies (inhibitors) to infused factor VIII
F8 gene and phenotype: single player in a team?
Anna Pavlova
Blood, 121(19), 3784-3785 (2013-05-11)
Innovations in coagulation: improved options for treatment of hemophilia A and B.
Ingrid Pabinger-Fasching et al.
Thrombosis research, 131 Suppl 2, S1-S1 (2013-03-30)
Puneet Gaitonde et al.
The Journal of biological chemistry, 288(24), 17051-17056 (2013-05-08)
Administration of recombinant factor VIII (FVIII), an important co-factor in blood clotting cascade, elicits unwanted anti-FVIII antibodies in hemophilia A (HA) patients. Previously, FVIII associated with phosphatidylserine (PS) showed significant reduction in the anti-FVIII antibody response in HA mice. The
Samantha C Gouw et al.
Blood, 121(20), 4046-4055 (2013-04-05)
The objective of this study was to examine the association of the intensity of treatment, ranging from high-dose intensive factor VIII (FVIII) treatment to prophylactic treatment, with the inhibitor incidence among previously untreated patients with severe hemophilia A. This cohort
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)